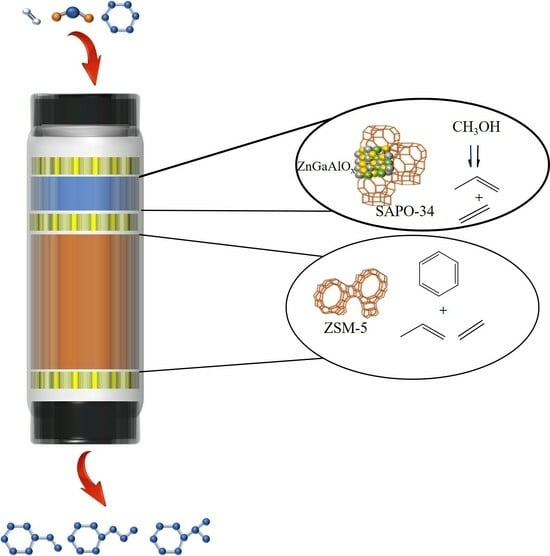
One-Step Production of Highly Selective Ethylbenzene and Propylbenzene from Benzene and Carbon Dioxide via Coupling Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Properties of Zn2Ga2−mAlmOx
2.2. Characterizations of SAPO-34 and ZSM-5
2.3. Catalytic Performance
2.3.1. Effects of Metal Oxides
2.3.2. Effects of the Catalyst Components Ratio
2.3.3. Effects of SARs of SAPO-34
2.3.4. Effects of SARs of ZSM-5
2.3.5. Stability Tests
2.4. Investigation of Propylbenzene Formation Sites
3. Materials and Methods
3.1. Source of Reagents
3.2. Synthesis
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, J.; Zhou, W.; Cheng, K.; Zhang, Q.; Kang, J.; Wang, Y. Highly Active ZnO-ZrO2 Aerogels Integrated with H-ZSM-5 for Aromatics Synthesis from Carbon Dioxide. ACS Catal. 2020, 10, 302–310. [Google Scholar] [CrossRef]
- Schieweck, B.G.; Jürling-Will, P.; Klankermayer, J. Structurally Versatile Ligand System for the Ruthenium Catalyzed One-Pot Hydrogenation of CO2 to Methanol. ACS Catal. 2020, 10, 3890–3894. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Qu, Y.; Liu, H.; Tang, C.; Miao, S.; Feng, Z.; An, H.; Li, C. Highly Selective Conversion of Carbon Dioxide to Lower Olefins. ACS Catal. 2017, 7, 8544–8548. [Google Scholar] [CrossRef]
- Feng, L.; Guo, S.; Yu, Z.; Cheng, Y.; Ming, J.; Song, X.; Cao, Q.; Zhu, X.; Wang, G.; Xu, D.; et al. Developing Multifunctional Fe-Based Catalysts for the Direct Hydrogenation of CO2 in Power Plant Flue Gas to Light Olefins. Catalysts 2024, 14, 204. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef]
- Fang, X.; Li, B.; Liu, H.; Xie, M.; Chen, Z.; Yang, L.; Han, J.; Zhu, W.; Liu, Z. High carbon utilization for CO2 conversion with chloromethane to aromatics over acidic zeolite catalyst. Chem Catal. 2023, 3, 100689. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, D.; He, L. Production of ethylbenzene and propylbenzene from benzene alkylation using carbon dioxide. Chem Catal. 2022, 2, 930–932. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, E.; Tang, J. Insight on Reaction Pathways of Photocatalytic CO2 Conversion. ACS Catal. 2022, 12, 7300–7316. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Cao, X.; Gao, L.; Li, J.; Li, L.; Xie, Y.; Zhao, Y.; Zhang, J.; Wu, M.; Liu, H. Electrochemical Carbon Dioxide Reduction to Ethylene: From Mechanistic Understanding to Catalyst Surface Engineering. Nano-Micro Lett. 2023, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Belgamwar, R.; Polshettiwar, V. Plasmonic Photocatalysis for CO2 Conversion to Chemicals and Fuels. ACS Mater. Lett. 2021, 3, 574–598. [Google Scholar] [CrossRef]
- Korologos, C.A.; Nikolaki, M.D.; Zerva, C.N.; Philippopoulos, C.J.; Poulopoulos, S.G. Photocatalytic oxidation of benzene, toluene, ethylbenzene and m-xylene in the gas-phase over TiO2-based catalysts. J. Photochem. Photobiol. A Chem. 2012, 244, 24–31. [Google Scholar] [CrossRef]
- Al-Sabahi, J.; Bora, T.; Al-Abri, M.; Dutta, J. Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS ONE 2017, 12, e0189276. [Google Scholar] [CrossRef] [PubMed]
- Tirumala, R.T.A.; Gyawali, S.; Wheeler, A.; Ramakrishnan, S.B.; Sooriyagoda, R.; Mohammadparast, F.; Khatri, N.; Tan, S.; Kalkan, A.K.; Bristow, A.D.; et al. Structure–Property–Performance Relationships of Cuprous Oxide Nanostructures for Dielectric Mie Resonance-Enhanced Photocatalysis. ACS Catal. 2022, 12, 7975–7985. [Google Scholar] [CrossRef]
- Mohammadparast, F.; Ramakrishnan, S.B.; Khatri, N.; Tirumala, R.T.A.; Tan, S.; Kalkan, A.K.; Andiappan, M. Cuprous Oxide Cubic Particles with Strong and Tunable Mie Resonances for Use as Nanoantennas. ACS Appl. Nano Mater. 2020, 3, 6806–6815. [Google Scholar] [CrossRef]
- Gyawali, S.; Tirumala, R.T.A.; Loh, H.; Andiappan, M.; Bristow, A.D. Photocarrier Recombination Dynamics in Highly Scattering Cu2O Nanocatalyst Clusters. J. Phys. Chem. C 2024, 128, 2003–2011. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, C.; Zhao, D.; Wang, Y.; Chu, W.; Feng, Z.; Zhu, X.; Xin, W.; Shang, Y.; Liu, S.; et al. Co-Crystalline ZSM-5/ZSM-11 Nanostructures for Alkylation of Benzene with Ethanol. ACS Appl. Nano Mater. 2021, 4, 10296–10306. [Google Scholar] [CrossRef]
- Mimura, N.; Saito, M. Dehydrogenation of ethylbenzene to styrene over Fe2O3/Al2O3 catalysts in the presence of carbon dioxide. Catal. Today 2000, 55, 173–178. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, C.; Wang, D.; He, J.; Sun, H.; Zhu, Z.; Yang, W. Shape-selective alkylation of benzene with ethylene over a core–shell ZSM-5@MCM-41 composite material. Chin. J. Chem. Eng. 2021, 37, 64–71. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Sun, H.; Zhang, B. Advances in development and industrial applications of ethylbenzene processes. Chin. J. Catal. 2016, 37, 16–26. [Google Scholar] [CrossRef]
- Gupta, R.; Uslu, H.; Majumder, S. Production of Styrene from Dehydrogenation of Ethylbenzene. Chem. Eng. Technol. 2022, 45, 817–823. [Google Scholar] [CrossRef]
- Perego, C.; Ingallina, P. Recent advances in the industrial alkylation of aromatics: New catalysts and new processes. Catal. Today 2002, 73, 3–22. [Google Scholar] [CrossRef]
- Gao, Y.; Neal, L.; Ding, D.; Wu, W.; Baroi, C.; Gaffney, A.M.; Li, F. Recent Advances in Intensified Ethylene Production—A Review. ACS Catal. 2019, 9, 8592–8621. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, K.; He, K.; Ma, X.; Dai, C. Direct and Highly Selective One-Step Production of Ethylbenzene from Syngas and Benzene. Ind. Eng. Chem. Res. 2023, 62, 15834–15843. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Dong, J.; Zhou, L.; Li, X.; Ge, H. Alkylbenzene synthesis from benzene and syngas over a ZnCrOx/beta bifunctional catalyst. React. Chem. Eng. 2022, 7, 1447–1460. [Google Scholar] [CrossRef]
- Safi, N.A.; Li, Y.; Yu, B.; Liu, P.; Wang, J.; Ge, H.; Zhang, K. The dependence of high catalytic performance on the tunable oxygen vacancy in the CZxS/Zn-HZSM-5 bifunctional catalyst for alkylation of benzene and syngas. Appl. Organomet. Chem. 2022, 36, e6744. [Google Scholar] [CrossRef]
- Feng, M.; Li, Y.; Liu, P.; Wang, J.; Ge, H.; Zhang, K.; Duan, D.; Wang, L.; Pei, X. A novel alkylation process of benzene with CO2 and H2 over bifunctional ZnxCeyZrzO/Z5 catalyst. Fuel 2022, 326, 125023. [Google Scholar] [CrossRef]
- Zhu, P.; Sun, J.; Yang, G.; Liu, G.; Zhang, P.; Yoneyama, Y.; Tsubaki, N. Tandem catalytic synthesis of benzene from CO2 and H2. Catal. Sci. Technol. 2017, 7, 2695–2699. [Google Scholar] [CrossRef]
- Zuo, J.; Liu, C.; Han, X.; Wen, D.; Liu, X.; Ye, L.; Zhuang, W.; Yuan, Y. Steering CO2 hydrogenation coupled with benzene alkylation toward ethylbenzene and propylbenzene using a dual-bed catalyst system. Chem Catal. 2022, 2, 1223–1240. [Google Scholar] [CrossRef]
- Huš, M.; Dasireddy, V.D.B.C.; Strah Štefančič, N.; Likozar, B. Mechanism. kinetics and thermodynamics of carbon dioxide hydrogenation to methanol on Cu/ZnAl2O4 spinel-type heterogeneous catalysts. Appl. Catal. B Environ. 2017, 207, 267–278. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, G.-F.; Zhao, F.; Gong, Z.-Z. First-principles study of the structural, mechanical and electronic properties of ZnX2O4 (X=Al, Cr and Ga). Chin. Phys. B 2011, 20, 047102. [Google Scholar] [CrossRef]
- Vasile, M.; Vlazan, P.; Avram, N.M. Characterization and optical properties of ZnGa2O4:Eu3+ nanophosphor grown by hydrothermal method. J. Alloys Compd. 2010, 500, 185–189. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhou, C.; Zhou, W.; Cheng, K.; Kang, J.; Zhang, Q.; Deng, W.; Wang, Y. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34. Chem. Commun. 2018, 54, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, T.; Sápi, A.; Ábel, M.; Farkas, F.; Gómez-Pérez, J.F.; Kukovecz, Á.; Kónya, Z. Ni–Zn–Al-Based Oxide/Spinel Nanostructures for High Performance, Methane-Selective CO2 Hydrogenation Reactions. Catal. Lett. 2019, 150, 1527–1536. [Google Scholar] [CrossRef]
- Kumar, N.; Kishan, H.; Rao, A.; Awana, V.P.S. Fe ion doping effect on electrical and magnetic properties of La0.7Ca0.3Mn1−xFexO3 (0 ≤ x ≤ 1). J. Alloys Compd. 2010, 502, 283–288. [Google Scholar] [CrossRef]
- Yang, F.; Fang, Y.; Liu, X.; Liu, X.; Muir, D.; Maclennan, A.; Zhu, X. One-Step Alkylation of Benzene with Syngas over Non-Noble Catalysts Mixed with Modified HZSM-5. Ind. Eng. Chem. Res. 2019, 58, 13879–13888. [Google Scholar] [CrossRef]
- Deng, X.; Lü, M.; Meng, J. Effect of heavy doping of nickel in compound Mo3Sb7: Structure and thermoelectric properties. J. Alloys Compd. 2013, 577, 183–188. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Nandhini, V.; Jeyaprakash, B.G. Improved sensing response of photo activated ZnO thin film for hydrogen peroxide detection. J. Colloid Interface Sci. 2016, 482, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Gurugubelli, T.R.; Babu, B.; Kim, J.; Yoo, K. Hydrothermal synthesis of Fe-doped ZnAl2O4 nanosheets: Bandgap engineering and room temperature ferromagnetism. Chem. Pap. 2021, 75, 6407–6416. [Google Scholar] [CrossRef]
- Akika, F.Z.; Benamira, M.; Lahmar, H.; Trari, M.; Avramova, I.; Suzer, Ş. Structural and optical properties of Cu-doped ZnAl2O4 and its application as photocatalyst for Cr(VI) reduction under sunlight. Surf. Interfaces 2020, 18, 100406. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, L.; Meng, F.; Wang, L.; Zhang, R.; Yang, G.; Li, Z. Boosting CO2 hydrogenation performance for light olefin synthesis over GaZrOx combined with SAPO-34. Appl. Catal. B Environ. 2022, 305, 121042. [Google Scholar] [CrossRef]
- Chen, J.-X.; Li, X.-X.; Ma, H.-P.; Huang, W.; Ji, Z.-G.; Xia, C.; Lu, H.-L.; Zhang, D.W. Investigation of the Mechanism for Ohmic Contact Formation in Ti/Al/Ni/Au Contacts to β-Ga2O3 Nanobelt Field-Effect Transistors. ACS Appl. Mater. Interfaces 2019, 11, 32127–32134. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Akatsuka, M.; Ozawa, A.; Kato, Y.; Kawaguchi, Y.; Yamamoto, M.; Tanabe, T.; Yoshida, T. Photocatalytic Activity of Ga2O3 Supported on Al2O3 for Water Splitting and CO2 Reduction. ACS Omega 2019, 4, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cheng, J.; Zhang, P.; Liu, X.; Zhuang, J.; Yu, Y.; Zhao, Q.; Wang, Y.; Zhu, X.; Yang, F. Examination of key factors determining the catalytic performance of Zn-Ga/HZSM-5 bifunctional catalysts and establishment of reaction network in alkylation of benzene with carbon dioxide. Appl. Catal. A Gen. 2022, 643, 118785. [Google Scholar] [CrossRef]
- Fu, L.; Liu, Y.; Hu, P.; Xiao, K.; Yu, G.; Zhu, D. Ga2O3 Nanoribbons: Synthesis, Characterization, and Electronic Properties. Chem. Mater. 2003, 15, 4287–4291. [Google Scholar] [CrossRef]
- Gao, P.; Dang, S.; Li, S.; Bu, X.; Liu, Z.; Qiu, M.; Yang, C.; Wang, H.; Zhong, L.; Han, Y.; et al. Direct Production of Lower Olefins from CO2 Conversion via Bifunctional Catalysis. ACS Catal. 2018, 8, 571–578. [Google Scholar] [CrossRef]
- Sood, A.; Tarntair, F.-G.; Wang, Y.-X.; Chang, T.-C.; Chen, Y.-H.; Liu, P.-L.; Horng, R.-H. Performance enhancement of ZnGa2O4 Schottky type deep-ultraviolet photodetectors by oxygen supercritical fluid treatment. Results Phys. 2021, 29, 104764. [Google Scholar] [CrossRef]
- He, H.; Lin, X.; Li, S.; Wu, Z.; Gao, J.; Wu, J.; Wen, W.; Ye, D.; Fu, M. The key surface species and oxygen vacancies in MnOx (0.4)-CeO2 toward repeated soot oxidation. Appl. Catal. B Environ. 2018, 223, 134–142. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Shi, D.; He, S.; Zhang, L.; Yan, W.; Qin, Z.; Li, J.; Dong, M.; Wang, J.; et al. Direct Conversion of Syngas into Light Olefins with Low CO2 Emission. ACS Catal. 2020, 10, 2046–2059. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the Influence of CeO2 Crystal Facet on CO2 Hydrogenation to Methanol over Pd/CeO2 Catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Wang, W.; Seiler, M.; Hunger, M. Role of Surface Methoxy Species in the Conversion of Methanol to Dimethyl Ether on Acidic Zeolites Investigated by in Situ Stopped-Flow MAS NMR Spectroscopy. J. Phys. Chem. B 2001, 105, 12553–12558. [Google Scholar] [CrossRef]
- Shen, C.; Bao, Q.; Xue, W.; Sun, K.; Zhang, Z.; Jia, X.; Mei, D.; Liu, C.-j. Synergistic effect of the metal-support interaction and interfacial oxygen vacancy for CO2 hydrogenation to methanol over Ni/In2O3 catalyst: A theoretical study. J. Energy Chem. 2022, 65, 623–629. [Google Scholar] [CrossRef]
- Ghavipour, M.; Al Hussami, R.; Levin, K.; Roy, R.; Kopyscinski, J. SAPO-34 Catalyst: Synthesis Optimization by Template Alteration and In Situ Coke Evolution Analysis in Methanol Conversion to Light Olefins. Ind. Eng. Chem. Res. 2023, 62, 18362–18378. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, H.; Xu, Z.; Qian, W.; Zhang, H.; Ying, W. Role of nanosized sheet-like SAPO-34 in bifunctional catalyst for syngas-to-olefins reaction. Fuel 2020, 273, 117771. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, W.-G.; So, J.-S.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G.; Sievers, C.; Sholl, D.S.; Nair, S.; et al. Propane dehydrogenation catalyzed by gallosilicate MFI zeolites with perturbed acidity. J. Catal. 2017, 345, 113–123. [Google Scholar] [CrossRef]
- Nan, Y.; Ma, S.; Zha, F.; Tian, H.; Tang, X.; Chang, Y.; Guo, X. Facile surfactant-assisted synthesis of nanosheet-like mesoporous SAPO-34 zeolites and catalysis performance for methanol to olefins. React. Kinet. Mech. Catal. 2022, 135, 1987–1998. [Google Scholar] [CrossRef]
- Yang, F.; Zhong, J.; Liu, X.; Zhu, X. A novel catalytic alkylation process of syngas with benzene over the cerium modified platinum supported on HZSM-5 zeolite. Appl. Energy 2018, 226, 22–30. [Google Scholar] [CrossRef]
- Potter, M.E.; Kezina, J.; Bounds, R.; Carravetta, M.; Mezza, T.M.; Raja, R. Investigating the role of framework topology and accessible active sites in silicoaluminophosphates for modulating acid-catalysis. Catal. Sci. Technol. 2018, 8, 5155–5164. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, Q.; Wang, J.; Ding, H.; Zhu, X.; Zhu, K.; Fan, W. Conquering the solubility barrier of di-n-octylamine as structure-directing agent in hierarchical silicoaluminophosphate synthesis by using phase-transfer synthesis. Chem. Eng. J. 2023, 461, 141887. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X.; Xue, W.; Ge, L.; Wang, T.; Qiu, M.; Wei, W.; Gao, P.; Sun, Y. Melting-assisted solvent-free synthesis of SAPO-11 for improving the hydroisomerization performance of n-dodecane. Chin. J. Catal. 2020, 41, 622–630. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, F.; Liu, X.; Liu, C.; Zhu, X. Performance of Bifunctional ZnZr/ZSM-5 Catalysts in the Alkylation of Benzene with Syngas. Catal. Lett. 2018, 148, 3618–3627. [Google Scholar] [CrossRef]
- Han, F.; Liu, H.; Cheng, W.; Xu, Q. Highly selective conversion of CO2 to methanol on the CuZnO-ZrO2 solid solution with the assistance of plasma. RSC Adv. 2020, 10, 33620–33627. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhang, C.; Liu, H.; Jin, Y.; Zhang, R. Enhanced performance of oxygen vacancies on CO2 adsorption and activation over different phases of ZrO2. Front. Energy 2023, 17, 545–554. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.; Mei, D.; Ge, Q. Active Oxygen Vacancy Site for Methanol Synthesis from CO2 Hydrogenation on In2O3(110): A DFT Study. ACS Catal. 2013, 3, 1296–1306. [Google Scholar] [CrossRef]
- Dahl, I.M.; Kolboe, S. On the reaction mechanism for propene formation in the MTO reaction over SAPO-34. Catal. Lett. 1993, 20, 329–336. [Google Scholar] [CrossRef]
- Tao, S.; Zhang, X.; Li, X.; Wang, Y.; Wang, B.; Yuan, Y.; Zhang, D.; Du, S.; Li, X. Hierarchical low-silica SAPO-34 with enhanced MTO performance: Mesopore template- and fluoride-free synthesis. Microporous Mesoporous Mater. 2023, 349, 112425. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]














| Sample | SBET/(m2·g−1) | Dpore/(nm) | Vtotal/(cm3·g−1) |
|---|---|---|---|
| ZnGaOx | 63 | 171 | 0.27 |
| Zn2Ga1.5Al0.5Ox | 70 | 136 | 0.24 |
| Zn2GaAlOx | 92 | 137 | 0.24 |
| Zn2Ga0.5Al1.5Ox | 53 | 104 | 0.18 |
| ZnAlOx | 50 | 98 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Guan, Y.; Wu, H.; Su, Z.; Zhuang, J.; Yan, S.; Zhu, X.; Yang, F. One-Step Production of Highly Selective Ethylbenzene and Propylbenzene from Benzene and Carbon Dioxide via Coupling Reaction. Catalysts 2024, 14, 288. https://doi.org/10.3390/catal14050288
Wang T, Guan Y, Wu H, Su Z, Zhuang J, Yan S, Zhu X, Yang F. One-Step Production of Highly Selective Ethylbenzene and Propylbenzene from Benzene and Carbon Dioxide via Coupling Reaction. Catalysts. 2024; 14(5):288. https://doi.org/10.3390/catal14050288
Chicago/Turabian StyleWang, Tianyun, Yingjie Guan, Haidan Wu, Zhaojie Su, Jianguo Zhuang, Siyan Yan, Xuedong Zhu, and Fan Yang. 2024. "One-Step Production of Highly Selective Ethylbenzene and Propylbenzene from Benzene and Carbon Dioxide via Coupling Reaction" Catalysts 14, no. 5: 288. https://doi.org/10.3390/catal14050288
APA StyleWang, T., Guan, Y., Wu, H., Su, Z., Zhuang, J., Yan, S., Zhu, X., & Yang, F. (2024). One-Step Production of Highly Selective Ethylbenzene and Propylbenzene from Benzene and Carbon Dioxide via Coupling Reaction. Catalysts, 14(5), 288. https://doi.org/10.3390/catal14050288