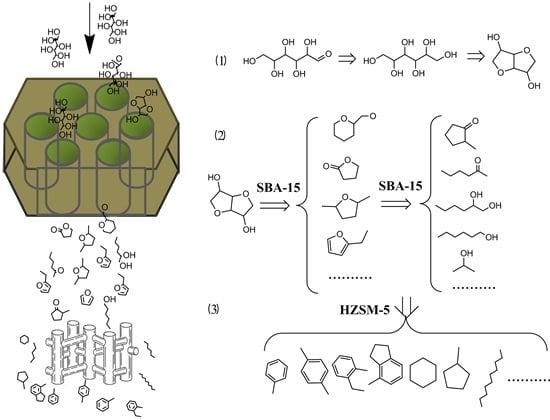
Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol over Ni-HZSM-5/SBA-15 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
| Samples | ABET (m2/g) a | Vm (cm3/g) a | Am (m2/g) a | Vmes (cm3/g) a | Dp (nm) a |
|---|---|---|---|---|---|
| HZSM-5 | 387.4 | 0.13 | 310.2 | 0.19 | / |
| SBA-15 | 506.0 | / | / | 0.70 | / |
| Ni-HZSM-5 | 341.7 | 0.09 | 227.2 | 0.17 | 17.8(22.5) b |
| Ni-SBA-15 | 402.0 | / | / | 0.55 | 6.1(9.3) b |
| Ni-HZSM-5/SBA-15 | 397.7 | 0.07 | 151.5 | 0.45 | 16.3(21.0) b |




| Samples | 373 K a | 573 K a | ||
|---|---|---|---|---|
| B (mmol/g) | L (mmol/g) | B (mmol/g) | L (mmol/g) | |
| Ni-SBA-15 b | 0.00 | 0.01 | 0.00 | 0.00 |
| HZSM-5 | 0.28 | 0.05 | 0.19 | 0.02 |
| Ni-HZSM-5 | 0.08 | 0.29 | 0.04 | 0.17 |
| Ni-HZSM-5/SBA-15 | 0.02 | 0.15 | 0.01 | 0.08 |

2.2. Products of Sorbitol Transformation
| Products (%) | HZSM-5 | Ni-HZSM-5 | Ni-SBA-15 | Ni-HZSM-5/SBA-15 |
|---|---|---|---|---|
| Over yields | ||||
| CO | / | 6.2 | 1.9 | 0.6 |
| CO2 | / | 1.9 | 2.1 | 2.8 |
| C1-C6 alkanes | 9.6 | 40.1 | 9.9 | 21.5 |
| Isosorbide | 56.9 | 7.9 | 30.4 | 3.2 |
| Ethanol | 3.1 | 1.3 | 6.5 | 2.5 |
| 2-Pentanone | 4.2 | 3.6 | 3.6 | 3.1 |
| 2-Hexanone | / | 1.0 | 1.2 | 1.3 |
| 3-Hexanone | / | / | 0.9 | 0.7 |
| 2-Methyl-cyclopentanone | / | 1.2 | 2.0 | 4.3 |
| 2-Ehyl-furan | 6.5 | 4.3 | 3.8 | 3.2 |
| 2,5-Dimethyl-furan | 3.3 | 3.6 | 3.4 | 3.0 |
| Oil | / | 22.4 | 12.3 | 40.4 |
| Total balance | 86.6 | 93.5 | 70.9 | 85.6 |
| Unidentified | 16.4 | 6.5 | 22.0 | 14.4 |
| Alkane selectivity | ||||
| C1 | / | 7.3 | 1.4 | 35.7 |
| C2 | 0.9 | 11.5 | 1.1 | 3.1 |
| C3 | 14.0 | 18.7 | 2.0 | 10.4 |
| C4 | 14.0 | 25.0 | 20.7 | 13.9 |
| C5 | 64.1 | 12.4 | 61.0 | 22.2 |
| C6 | 7.0 | 25.1 | 6.6 | 14.6 |
| Oil selectivity | ||||
| Toluene | / | 8.9 | 3.6 | 4.7 |
| Xylenes | / | 19.2 | 4.0 | 20.3 |
| Alkylbenzenes | / | 20.5 | 6.0 | 15.9 |
| Indenes | / | 10.1 | / | 8.6 |
| Naphthalenes | / | 20.2 | / | 20.2 |
| Decane | / | / | 4.3 | 0.1 |
| Undecane | / | / | 4.1 | 0.7 |
| Dodecane | / | / | 6.1 | 1.1 |
| Branched-alkanes | / | 0.5 | 10.5 | 3.1 |
| Methyl-cyclopentane | / | 4.0 | 19.8 | 5.9 |
| Cyclohexane | 2.0 | 16.2 | 1.1 | |
| Methyl-cyclohexane | 1.6 | 12.1 | 1.0 | |
| Ethyl-cyclohexane | 0.5 | 9.6 | 2.3 | |
| Oxy-compounds | / | 10.2 | 13.0 | 11.7 |
| Others | / | 2.0 | 9.6 | 3.3 |
| Catalyst | C% | H% | O% | C:H |
|---|---|---|---|---|
| Ni-HZSM-5 | 80.4 | 9.0 | 9.6 | 8.9 |
| Ni@HZSM-5/SBA-15 | 86.5 | 10.1 | 3.4 | 8.6 |

2.3. Reaction Network

3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Tests
3.4. Product Analysis
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, T.; Qiu, S.; Weng, Y.; Chen, L.; Liu, Q.; Long, J.; Tan, J.; Zhang, Q.; Zhang, Q.; Ma, L. Liquid fuel production by aqueous phase catalytic transformation of biomass for aviation. Appl. Energy 2015, 160, 329–335. [Google Scholar] [CrossRef]
- Sacia, E.R.; Balakrishnan, M.; Deaner, M.H.; Goulas, K.A.; Toste, F.D.; Bell, A.T. Highly selective condensation of biomass-derived methyl ketones as a source of aviation fuel. ChemSusChem 2015, 8, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.A.Z.A.K.K. Activity of Oxalic Acid Treated ZnO–CuO-HZSM-5 Catalyst for the Transformation of Methanol to Gasoline Range Hydrocarbons. Ind. Eng. Chem. Res. 2008, 47, 2970–2975. [Google Scholar]
- Grilc, M.; Veryasov, G.; Likozar, B.; Jesih, A.; Levec, J. Hydrodeoxygenation of solvolysed lignocellulosic biomass by unsupported MoS2, MoO2, Mo2C and WS2 catalysts. Appl. Catal. B 2015, 163, 467–477. [Google Scholar] [CrossRef]
- Kim, Y.T.; Dumesic, J.A.; Huber, G.W. Aqueous-phase hydrodeoxygenation of sorbitol: A comparative study of Pt/Zr phosphate and PtReOx/C. J. Catal. 2013, 304, 72–85. [Google Scholar] [CrossRef]
- Dietrich, P.J.; Lobo-Lapidus, R.J.; Wu, T.P.; Sumer, A.; Akatay, M.C.; Fingland, B.R.; Guo, N.; Dumesic, J.A.; Marshall, C.L.; Stach, E.; et al. Aqueous Phase Glycerol Reforming by PtMo Bimetallic Nano-Particle Catalyst: Product Selectivity and Structural Characterization. Top. Catal. 2012, 55, 53–69. [Google Scholar] [CrossRef]
- Kirilin, A.V.; Tokarev, A.V.; Kustov, L.M.; Salmi, T.; Mikkola, J.P.; Murzin, D.Y. Aqueous phase reforming of xylitol and sorbitol: Comparison and influence of substrate structure. Appl. Catal. A 2012, 435, 172–180. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, T.; Li, B.; Jiang, T.; Ma, L.; Zhang, X.; Liu, Q. Aqueous phase reforming of sorbitol to bio-gasoline over Ni/HZSM-5 catalysts. Appl. Energy 2012, 97, 509–513. [Google Scholar] [CrossRef]
- Li, N.; Huber, G.W. Aqueous-phase hydrodeoxygenation of sorbitol with Pt/SiO2–Al2O3: Identification of reaction intermediates. J. Catal. 2010, 270, 48–59. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Q.; Xie, Z.; Yang, W.; Li, C. The roles of acidity and structure of zeolite for catalyzing toluene alkylation with methanol to xylene. Microporous Mesoporous Mater. 2006, 88, 16–21. [Google Scholar] [CrossRef]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Zhao, K.; Wu, H.; Wang, X.; He, F.; Li, H. Maximum synergistic effect in the coupling conversion of bio-derived furans and methanol over ZSM-5 for enhancing aromatic production. Green Chem. 2014, 16, 2580–2586. [Google Scholar] [CrossRef]
- Street, J.; Yu, F.; Wooten, J.; Columbus, E.; White, M.G.; Warnock, J. Gasoline-range hydrocarbon production using biomass derived synthesis gas over Mo/H+ZSM-5. Fuel 2012, 96, 239–249. [Google Scholar] [CrossRef]
- Simonetti, D.A.; Kunkes, E.L.; Dumesic, J.A. Gas-phase conversion of glycerol to synthesis gas over carbon-supported platinum and platinum-rhenium catalysts. J. Catal. 2007, 247, 298–306. [Google Scholar] [CrossRef]
- Hoang, T.Q.; Zhu, X.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Effects of HZSM-5 crystallite size on stability and alkyl-aromatics product distribution from conversion of propanal. Catal. Commun. 2010, 11, 977–981. [Google Scholar] [CrossRef]
- Yin, C.; Zhao, R.; Liu, C. Transformation of olefin over Ni/HZSM-5 catalyst. Fuel 2005, 84, 701–706. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Liu, Q.; Wang, T.; Zhang, Q.; Ma, L. A simple method to prepare highly active and dispersed Ni/MCM-41 catalysts by co-impregnation. Catal. Commun. 2013, 42, 73–78. [Google Scholar] [CrossRef]
- Krishnan, C.K.; Nakamura, K.; Hirata, H.; Ogura, M. Pt/CeO2–ZrO2 present in the mesopores of SBA-15—A better catalyst for CO oxidation. Phys. Chem. Chem. Phys. 2010, 12, 7513–7520. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.Q.; Zhu, X.; Danuthai, T.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Conversion of Glycerol to Alkyl-aromatics over Zeolites. Energy Fuels 2010, 24, 3804–3809. [Google Scholar] [CrossRef]
- Murata, M.I.K.; Takahara, I. Effects of surface modification of HZSM-5 catalysts on direct transformation of ethanol into lower olefins. J. Jpn. Pet. Inst. 2008, 51, 234–239. [Google Scholar] [CrossRef]
- Zhu, X.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Tailoring the mesopore structure of HZSM-5 to control product distribution in the conversion of propanal. J. Catal. 2010, 271, 88–98. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, T.; Zuo, Y.; Guo, Q.X.; Fu, Y. Production of aromatic hydrocarbons through catalytic pyrolysis of 5-hydroxymethylfurfural from biomass. Bioresour. Technol. 2013, 147, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, J.; Wang, J.; Zhang, L.; Gao, J.; Liu, A. Catalytic performance and characterization of Ni-doped HZSM-5 catalysts for selective trimerization of n-butene. Fuel Process. Technol. 2009, 90, 863–870. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Jin, B.; Xiao, G.; Chen, R. Biomass catalytic pyrolysis to produce olefins and aromatics with a physically mixed catalyst. Bioresour. Technol. 2013, 140, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, T.J.; Ma, L.L.; Li, Y.P.; Zhang, Q.; Zhang, X.H. Investigation on the xylitol aqueous-phase reforming performance for pentane production over Pt/HZSM-5 and Ni/HZSM-5 catalysts. Appl. Energy 2012, 90, 51–57. [Google Scholar] [CrossRef]
- Wenga, Y.; Qiu, S.; Xu, Y.; Ding, M.; Chen, L.; Zhang, Q.; Ma, L.; Wang, T. One-pot aqueous phase catalytic conversion of sorbitol to gasoline over nickel catalyst. Energy Convers. Manag. 2015, 94, 95–102. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Gayubo, A.G.; Atutxa, A.; Valle, B.; Bilbao, J. Regeneration of a HZSM-5 zeolite catalyst deactivated in the transformation of aqueous ethanol into hydrocarbons. Catal. Today 2005, 107–108, 410–416. [Google Scholar] [CrossRef]
- Carlson, T.R.; Jae, J.; Lin, Y.-C.; Tompsett, G.A.; Huber, G.W. Catalytic fast pyrolysis of glucose with HZSM-5: The combined homogeneous and heterogeneous reactions. J. Catal. 2010, 270, 110–124. [Google Scholar] [CrossRef]
- Vilcocq, L.; Cabiac, A.; Especel, C.; Lacombe, S.; Duprez, D. New insights into the mechanism of sorbitol transformation over an original bifunctional catalytic system. J. Catal. 2014, 320, 16–25. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Gurbuz, E.I.; Dumesic, J.A. Vapour-phase C–C coupling reactions of biomass-derived oxygenates over Pd/CeZrOx catalysts. J. Catal. 2009, 266, 236–249. [Google Scholar] [CrossRef]
- Bond, J.Q.; Upadhye, A.A.; Olcay, H.; Tompsett, G.A.; Jae, J.; Xing, R.; Alonso, D.M.; Wang, D.; Zhang, T.; Kumar, R.; et al. Production of renewable jet fuel range alkanes and commodity chemicals from integrated catalytic processing of biomass. Energy Environ. Sci. 2014, 7, 1500–1523. [Google Scholar] [CrossRef]
- Blommel, P.G.; Keenan, G.R.; Rozmiarek, R.T.; Cortrigth, R.D. Catalytic conversion of sugar into conventional gasoline, diesel, jet fuel, and other hydrocarbons. Int. Sugar J. 2008, 110, 672–681. [Google Scholar]
- Zhang, Q.; Jiang, T.; Li, B.; Wang, T.; Zhang, X.; Zhang, Q.; Ma, L. Highly Selective Sorbitol Hydrogenolysis to Liquid Alkanes over Ni/HZSM-5 Catalysts Modified with Pure Silica MCM-41. ChemCatChem 2012, 4, 1084–1087. [Google Scholar] [CrossRef]
- Qiu, S.B.; Weng, Y.J.; Li, Y.P.; Ma, L.L.; Zhang, Q.; Wang, T.J. Promotion of Ni/MCM-41 Catalyst for Hydrogenation of Naphthalene by co-Impregnation with Polyols. Chin. J. Chem. Phys. 2014, 27, 433–438. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Tian, S.; Zhao, E.; Zhang, J.; Liu, C. Preparation of bifunctional NiPb/ZnO-diatomite-ZSM-5 catalyst and its reactive adsorption desulfurization coupling aromatization performance in FCC gasoline upgrading process. Fuel 2013, 104, 201–207. [Google Scholar] [CrossRef]
- Vu, X.H.; Nguyen, S.; Dang, T.T.; Phan, B.M.Q.; Nguyen, D.A.; Armbruster, U.; Martin, A. Catalytic Cracking of Triglyceride-Rich Biomass toward Lower Olefins over a Nano-ZSM-5/SBA-15 Analog Composite. Catalysts 2015, 5, 1692–1703. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Y.; Qiu, S.; Ma, L.; Liu, Q.; Ding, M.; Zhang, Q.; Zhang, Q.; Wang, T. Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol over Ni-HZSM-5/SBA-15 Catalyst. Catalysts 2015, 5, 2147-2160. https://doi.org/10.3390/catal5042147
Weng Y, Qiu S, Ma L, Liu Q, Ding M, Zhang Q, Zhang Q, Wang T. Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol over Ni-HZSM-5/SBA-15 Catalyst. Catalysts. 2015; 5(4):2147-2160. https://doi.org/10.3390/catal5042147
Chicago/Turabian StyleWeng, Yujing, Songbai Qiu, Longlong Ma, Qiying Liu, Mingyue Ding, Qian Zhang, Qi Zhang, and Tiejun Wang. 2015. "Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol over Ni-HZSM-5/SBA-15 Catalyst" Catalysts 5, no. 4: 2147-2160. https://doi.org/10.3390/catal5042147
APA StyleWeng, Y., Qiu, S., Ma, L., Liu, Q., Ding, M., Zhang, Q., Zhang, Q., & Wang, T. (2015). Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol over Ni-HZSM-5/SBA-15 Catalyst. Catalysts, 5(4), 2147-2160. https://doi.org/10.3390/catal5042147