Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship
Abstract
:1. Introduction
2. Results
2.1. Characterization Studies
2.1.1. Textural Characterization (BET Analysis)
2.1.2. Structural Characterization (XRD Analysis)
2.1.3. Reducibility Studies (H2-TPR)
2.1.4. Surface Characterization (XPS Analysis)
2.2. Catalytic Activity Studies
2.2.1. Effect of Metal Entity on Steam Reforming Performance
2.2.2. Effect of Metal Loading
2.2.3. Effect of S/C Ratio
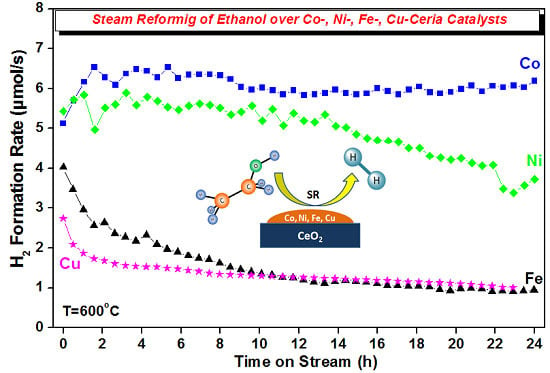
2.2.4. Stability Experiments
3. Discussion
4. Materials and Methods
4.1. Materials Synthesis
4.2. Characterization Studies
4.2.1. Textural Characterization (BET)
4.2.2. Structural Characterization
4.2.3. Morphological Characterization
4.2.4. Redox Characterization (H2-TPR)
4.2.5. Surface Characterization (XPS)
4.3. Catalytic Activity Measurements
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goltsov, V.A.; Veziroglu, T.N.; Goltsova, L.F. Hydrogen civilization of the future—A new conception of the IAHE. Int. J. Hydrogen Energy 2006, 31, 153–159. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Sumathy, K.; Leung, D.Y.C. Potential of renewable hydrogen production for energy supply in Hong Kong. Int. J. Hydrogen Energy 2006, 31, 1401–1412. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G.A. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Dien, B.S.; Cotta, M.A.; Jeffries, T.W. Bacteria engineered for fuel ethanol production: Current Status. Appl. Microbiol. Biotechnol. 2003, 63, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J.Y. Hydrolysis of lignocellulosic materials for ethanol production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; van Veen, A.C.; Provendier, H.; Mirodatos, C.; Shen, W. Autothermal reforming of ethanol for hydrogen production over an Rh/CeO2 catalyst. Catal. Today 2008, 138, 152–156. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrogen Energy 2006, 32, 2367–2373. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Matsumoto, T.; Kanai, H.; Utani, K.; Matsumura, Y.; Shen, W.J.; Imamura, S. Catalytic steam reforming of ethanol to produce hydrogen and acetone. Appl. Catal. A 2005, 279, 273–277. [Google Scholar] [CrossRef]
- Contreras, J.L.; Salmones, J.; Colin-Luna, J.A.; Nuno, L.; Quintana, B.; Cordova, I.; Zeifert, B.; Tapia, C.; Fuentes, G.A. Catalysts for H2 production using the ethanol steam reforming (a review). Int. J. Hydrogen Energy 2014, 39, 18835–18853. [Google Scholar] [CrossRef]
- Haryanto, H.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts. Catal. Commun. 2004, 5, 611–615. [Google Scholar] [CrossRef]
- Erdohelyi, A.; Rasko, J.; Kecskes, T.; Toth, M.; Domok, M.; Baan, K. Hydrogen formation in ethanol reforming on supported noble metal catalysts. Catal. Today 2006, 116, 367–376. [Google Scholar] [CrossRef]
- Liguras, D.K.; Kondarides, D.I.; Verykios, X.E. Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts. Appl. Catal. B 2003, 43, 345–354. [Google Scholar] [CrossRef]
- Cavallaro, S.; Chiodo, V.; Freni, S.; Mondello, N.; Frusteri, F. Performance of Rh/Al2O3 catalyst in the steam reforming of ethanol: H2 Production for MCFC. Appl. Catal. A 2003, 249, 119–128. [Google Scholar] [CrossRef]
- Diagne, C.; Idriss, H.; Pearson, K.; Gomez-Garcia, M.A.; Kiennemann, A.R. Efficient hydrogen production by ethanol reforming over Rh catalysts effect of addition of Zr on CeO2 for the oxidation of CO to CO2. C.R. Chim. 2004, 7, 617–622. [Google Scholar] [CrossRef]
- Mathure, P.V.; Ganguly, S.; Patwardhan, A.V.; Saha, R.K. Steam reforming of ethanol using a commercial nickel-based catalyst. Ind. Eng. Chem. Res. 2007, 46, 8471–8479. [Google Scholar] [CrossRef]
- Konsolakis, M.; Ioakeimidis, Z. Surface/structure functionalization of copper-based catalysts by metal-support and/or metal–metal interactions. Appl. Surf. Sci. 2014, 320, 244–255. [Google Scholar] [CrossRef]
- Gates, S.M.; Russell, J.N.; Yates, J.T. Bond activation sequence observed in the chemisorption and surface reaction of ethanol on Ni(lll). Surf. Sci. 1986, 171, 111–134. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Patel, M.; Jindal, T.K.; Pant, K.K. Kinetic Study of steam reforming of ethanol on Ni-Based ceria—Zirconia catalyst. Ind. Eng. Chem. Res. 2013, 52, 15763–15771. [Google Scholar] [CrossRef]
- Homs, N.; Llorca, J.; Ramírez de la Piscina, P. Low-temperature steam-reforming of ethanol over ZnO-supported Ni and Cu catalysts: The Effect of Nickel and Copper Addition to ZnO-Supported Cobalt-Based Catalysts. Catal. Today 2006, 116, 361–336. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.J.; Stach, E.A.; Rodriguez, J.A. Steam reforming of ethanol on Ni/CeO2: Reaction Pathway and Interaction between Ni and the CeO2 Support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Evans, J.; Agnoli, S.; Barrio, L.; Chen, T.-L.; Hrbek, J.; Rodriguez, J.A. Water–gas shift and CO methanation reactions over Ni–CeO2(111) catalysts. Top. Catal. 2011, 54, 34–41. [Google Scholar] [CrossRef]
- Carrasco, J.; Lopez-Duran, D.; Liu, Z.; Duchon, T.; Evans, J.; Senanayake, S.D.; Crumlin, E.J.; Matolin, V.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. In situ and theoretical studies for the dissociation of water on an active Ni/CeO2 catalyst: Importance of Strong Metal–Support Interactions for the Cleavage of O–H Bonds. Angew. Chem. Int. Ed. 2015, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulou, K.; Kalamaras, C.M.; Efstathiou, A.M. Ceria-Based Materials for Hydrogen Production Via Hydrocarbon Steam Reforming and Water-Gas Shift Reactions. Rec. Pat. Mat. Sci. 2011, 4, 122–145. [Google Scholar] [CrossRef]
- Ilieva, L.; Pantaleo, G.; Sobczak, J.W.; Ivanov, I.; Venezia, A.M.; Andreev, D. NO reduction by CO in the presence of water over gold supported catalysts on CeO2—Al2O3 mixed support, prepared by mechanochemical activation. Appl. Catal. B 2007, 76, 107–114. [Google Scholar] [CrossRef]
- Baudin, F.; da Costa, P.; Thomas, C.; Calvo, S.; Lendresse, Y.; Schneider, S.; Delacroix, F.; Plassat, G.; Mariadassou, G.D. NOx reduction over CeO2-ZrO2 supported iridium catalyst in the presence of propanol. Top. Catal. 2004, 30/31, 97–101. [Google Scholar] [CrossRef]
- De Lima, S.M.; da Cruz, I.O.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Pt/CeZrO2 catalyst. J. Catal. 2008, 257, 356–368. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic steam reforming of ethanol over high surface area CeO2: The Role of CeO2 as an Internal Pre-Reforming Catalyst. Appl. Catal. B 2006, 66, 29–39. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Li, M.; Wang, S.; Ma, X.; Gong, J. Enhanced Oxygen Mobility and Reactivity for Ethanol Steam Reforming. AIChE J. 2012, 58, 516–525. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, L.; Qin, Y. Study on the Carbon Deposition in Steam Reforming of Ethanol over Co/CeO2 Catalyst. Chem. Eng. J. 2008, 145, 25–31. [Google Scholar] [CrossRef]
- Song, H.; Ozkan, U.S. Changing the Oxygen Mobility in Co/Ceria Catalysts by Ca Incorporation: Implications for Ethanol Steam Reforming. J. Phys. Chem. A 2010, 114, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Ozkan, U.S. Ethanol steam reforming over Co-based catalysts: Role of Oxygen Mobility. J. Catal. 2009, 261, 66–74. [Google Scholar] [CrossRef]
- Llorca, J.; Homs, N.; Sales, J.; Ramirez de la Piscina, P. Efficient production of hydrogen over supported cobalt catalysts from Ethanol Steam Reforming. J. Catal. 2002, 209, 306–317. [Google Scholar] [CrossRef]
- Llorca, J.; Ramirez de la Piscina, P.; Dalmonb, J.-A.; Sales, J.; Homs, N. CO-free hydrogen from steam-reforming of bioethanol over ZnO-supported cobalt catalysts. Effect of the metallic precursor. Appl. Catal. B 2003, 43, 355–369. [Google Scholar] [CrossRef]
- Casanovas, A.; Roig, M.; de Leitenburg, C.; Trovarelli, A.; Llorca, J. Ethanol steam reforming and water gas shift over Co/ZnO catalytic honeycombs doped with Fe, Ni, Cu, Cr and Na. Int. J. Hydrogen Energy 2010, 35, 7690–7698. [Google Scholar] [CrossRef]
- Song, H.; Zhang, L.; Watson, R.B.; Braden, D.; Ozkan, U.S. Investigation of bio-ethanol steam reforming over cobalt-based catalysts. Catal. Today 2007, 129, 346–354. [Google Scholar] [CrossRef]
- Soykal, I.I.; Sohn, H.; Ozkan, U.S. Effect of Support Particle Size in Steam Reforming of Ethanol over Co/CeO2 Catalysts. ACS Catal. 2012, 2, 2335–2348. [Google Scholar] [CrossRef]
- Espinal, R.; Taboada, E.; Molins, E.; Chimentao, R.J.; Medina, F.; Llorca, J. Cobalt hydrotalcites as catalysts for bioethanol steam reforming. The promoting effect of potassium on catalyst activity and long-term stability. Appl. Catal. B 2012, 127, 59–67. [Google Scholar] [CrossRef]
- Espinal, R.; Anzola, A.; Adrover, E.; Roig, M.; Chimentao, R.; Medina, F.; Lopez, E.; Borio, D.; Llorca, J. Durable ethanol steam reforming in a catalytic membrane reactor at moderate temperature over cobalt hydrotalcite. Int. J. Hydrogen Energy 2014, 39, 10902–10910. [Google Scholar] [CrossRef]
- Espinal, R.; Taboada, E.; Molins, E.; Chimentao, R.J.; Medina, F.; Llorca, J. Ethanol Steam reforming over hydrotalcite-derived Co Catalysts doped with Pt and Rh. Top. Catal. 2013, 56, 1660–1671. [Google Scholar] [CrossRef]
- Ferencz, Z.; Erdőhelyi, A.; Baán, K.; Oszkó, A.; Óvári, L.; Kónya, Z.; Papp, C.; Steinrück, H.-P.; Kiss, J. Effects of support and Rh additive on Co-based catalysts in the ethanol steam reforming reaction. ACS Catal. 2014, 4, 1205–1218. [Google Scholar] [CrossRef]
- Varga, E.; Ferencz, Z.; Oszkó, A.; Erdőhelyi, A.; Kiss, J. Oxidation states of active catalytic centers in ethanol steam reforming reaction on ceria based Rh promoted Co catalysts: An XPS Study. J. Mol. Catal. A 2015, 397, 127–133. [Google Scholar] [CrossRef]
- Gamarra, D.; Munuera, G.; Hungría, A.B.; Fernández-García, M.; Conesa, J.C.; Midgley, P.A.; Wang, X.Q.; Hanson, J.C.; Rodríguez, J.A.; Martínez-Arias, A. Structure-activity relationship in nanostructured copper-ceria-based preferential CO oxidation catalysts. J. Phys. Chem. C 2007, 111, 11026–11038. [Google Scholar] [CrossRef]
- Al-Musa, A.; Al-Saleh, M.; Ioakimidis, Z.; Ouzounidou, M.; Yentekakis, I.V.; Konsolakis, M.; Marnellos, G.E. Hydrogen production by iso-octane steam reforming over Cu catalysts supported on Rare Earth Oxides (REOs). Int. J. Hydrogen Energy 2014, 39, 1350–1363. [Google Scholar] [CrossRef]
- Breen, B.; Ross, J.R.H. Methanol reforming for fuel-cell applications: Development of Zirconia-Containing Cu-Zn-Al Catalysts. Catal. Today 1999, 511, 521–533. [Google Scholar] [CrossRef]
- Turco, M.; Bagnasco, G.; Cammarano, C.; Senese, P.; Costantino, U.; Sisani, M. Cu/ZnO/Al2O3 catalysts for oxidative steam reforming of methanol: The Role of Cu and the Dispersing Oxide Matrix. Appl. Catal. B 2007, 77, 46–57. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Li, Y.; Xu, Y.; Xin, Q.; Shen, W. CuO/CeO2 catalysts: Redox Features and Catalytic Behaviors. Appl. Catal. A 2005, 288, 116–125. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Effect of dopants on the performance of CuO-CeO2 catalysts in methanol steam reforming. Appl. Catal. B 2007, 69, 226–234. [Google Scholar] [CrossRef]
- Konsolakis, M.; Sgourakis, M.; Carabineiro, S.A.C. Surface and redox properties of cobalt–ceria binary oxides: On the Effect of Co Content and Pretreatment Conditions. Appl. Surf. Sci. 2015, 341, 48–54. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Wu, G.; Gong, J. Synthesis of stable Ni-CeO2 catalysts via ball-milling for ethanol steam reforming. Catal. Today 2014, 233, 53–60. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Can, L. Reduction property and catalytic activity of Ce1-xNixO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Cai, W.; Xu, Y.; Shen, W. Steam reforming of bio-ethanol for the production of hydrogen over ceria-supported Co, Ir and Ni catalysts. Catal. Commun. 2006, 7, 367–372. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Donato, S.; Bonura, G.; Cavallaro, S. Steam and auto-thermal reforming of bio-ethanol over MgO and CeO2 Ni supported catalysts. Int. J. Hydrogen Energy 2006, 31, 2193–2199. [Google Scholar] [CrossRef]
- Perez-Alonso, F.J.; Melián-Cabrera, I.; López Granados, M.; Kapteijn, F.; Fierro, J.L.G. Synergy of FexCe1-xO2 mixed oxides for N2O decomposition. J. Catal. 2006, 239, 340–346. [Google Scholar] [CrossRef]
- Jin, Y.; Datye, A.K. Phase Transformations in Iron Fischer-Tropsch catalysts during temperature-programmed reduction. J. Catal. 2000, 196, 8–17. [Google Scholar] [CrossRef]
- Voskoboinikov, T.V.; Chen, H.Y.; Sachtler, W.M.H. On the nature of active sites in Fe/ZSM-5 catalysts for NOx abatement. Appl. Catal. B 1998, 19, 279–287. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Ascaso, S.; Stelmachowsk, P.; Legutko, P.; Kotarba, A.; Moliner, R.; Lázaro, M.J. Influence of the surface potassium species in Fe-K/Al2O3 catalysts on the soot oxidation activity in the presence of NOx. Appl. Catal. B 2014, 152–153, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Reddy, G.K.; Boolchand, P.; Smirniotis, P.G. Sulfur tolerant metal doped Fe/Ce catalysts for high temperature WGS reaction at low steam to CO ratios—XPS and Mössbauer spectroscopic study. J. Catal. 2011, 282, 258–269. [Google Scholar] [CrossRef]
- Zyryanova, M.M.; Snytnikov, P.V.; Gulyaev, R.V.; Amosov Yu, I.; Boronin, A.I.; Sobyanin, V.A. Performance of Ni/CeO2 catalysts for selective CO methanation in hydrogen-rich gas. Chem. Eng. J. 2014, 238, 189–197. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 2000, 27, 357–366. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Kundakovic, L.; Flytzani-Stephanopoulos, M. Reduction characteristics of copper oxide in cerium and zirconium oxide systems. Appl. Catal. A 1998, 171, 13–29. [Google Scholar]
- Li, W.; Flytzani-Stephanopoulos, M. Total Oxidation of Carbon-Monoxide and Methane over Transition Metal Fluorite Oxide Composite Catalysts: II. Catalyst Characterization and Reaction-Kinetics. J. Catal. 1995, 153, 317–332. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Gurbani, A.; González-Marcos, M.P.; Gutiérrez-Ortiz, M.A. Selective CO oxidation in H2 streams on CuO/CexZr1-xO2 catalysts: Correlation between Activity and Low Temperature Reducibility. Int. J. Hydrogen Energy 2012, 37, 1993–2006. [Google Scholar] [CrossRef]
- Lamonier, C.; Bennani, A.; D’Huysser, A.; Aboukaïs, A.; Wrobel, G. Evidence for different copper species in precursors of copper-cerium oxide catalysts for hydrogenation reactions: An X-Ray Diffraction, EPR and X-Ray Photoelectron Spectroscopy Study. Faraday Trans. 1996, 92, 131–136. [Google Scholar] [CrossRef]
- Mai, H.; Zhang, D.; Shi, L.; Li, H. Highly active Ce1-xCuxO2 nanocomposite catalysts for the low temperature oxidation of CO. Appl. Surf. Sci. 2011, 257, 7551–7559. [Google Scholar] [CrossRef]
- Rao, K.N.; Venkataswamy, P.; Reddy, B.M. Structural Characterization and Catalytic Evaluation of Supported Copper–Ceria Catalysts for Soot Oxidation. Ind. Eng. Chem. Res. 2011, 50, 11960–11969. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Bakker, J.J.W.; Soares, O.S.G.P.; Chen, X.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L.; Gascon, J.; Kapteijn, F. Stabilized gold on cerium-modified cryptomelane: Highly Active in Low-Temperature CO Oxidation. J. Catal. 2014, 309, 58–65. [Google Scholar] [CrossRef]
- Varga, E.; Pusztai, P.; Óvári, L.; Oszkó, A.; Erdőhelyi, A.; Papp, C.; Steinrück, H.-P.; Kónya, Z.; Kiss, J. Probing the interaction of Rh, Co and bimetallic Rh–Co nanoparticles with the CeO2 support: Catalytic Materials for Alternative Energy Generation. Phys. Chem. Chem. Phys. 2015, 17, 27154–27166. [Google Scholar] [CrossRef] [PubMed]
- Vári, G.; Óvári, L.; Papp, C.; Steinrück, H.-P.; Kiss, J.; Kónya, Z. The Interaction of Cobalt with CeO2(111) Prepared on Cu(111). J. Phys. Chem. C 2015, 119, 9324–9333. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Roger, A.C. Methanation of carbon dioxide over nickel-based Ce0.72Zr0.28O2 mixed oxide catalysts prepared by sol–gel method. Appl. Catal. A 2009, 369, 90–96. [Google Scholar] [CrossRef]
- Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G. CeO2-supported Pt/Ni catalyst for the renewable and clean H2 production via ethanol steam reforming. Appl. Catal. B Environ. 2013, 145, 73–84. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; Vizcaino, A.J. Hydrogen production by ethanol steam reforming over Cu-Ni/SBA-15 supported catalysts prepared by direct synthesis and impregnation. Appl. Catal. A 2007, 327, 82–94. [Google Scholar] [CrossRef]
- Pang, X.; Chen, Y.; Dai, R.; Cui, P. Co/CeO2 Catalysts Prepared Using Citric Acid Complexing for Ethanol Steam Reforming. Chin. J. Catal. 2012, 33, 281–289. [Google Scholar] [CrossRef]
- Fatsikostas, A.N.; Verykios, X.E. Reaction network of steam reforming of ethanol over Ni-based catalysts. J. Catal. 2004, 225, 439–452. [Google Scholar] [CrossRef]
- Fierro, V.; Akdim, O.; Mirodatos, C. On-board hydrogen production in a hybrid electric vehicle by bio-ethanol oxidative steam reforming over Ni and noble metal based catalysts. Green Chem. 2003, 5, 20–24. [Google Scholar] [CrossRef]
- Mariño, F.; Boveri, M.; Baronetti, G.; Laborde, M. Hydrogen production from steam reforming of bioethanol using Cu/Ni/K/γ-Al2O3 catalysts. Effect of Ni. Int. J. Hydrogen Energy 2001, 26, 665–668. [Google Scholar] [CrossRef]
- Melnick, J.G.; Radosevich, A.T.; Villagrán, D.; Nocera, D.G. Decarbonylation of ethanol to methane, carbon monoxide and hydrogen by a [PNP]Ir complex. Chem. Commun. 2010, 46, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Agus, H.; Sandun, F.; Naveen, M.; Sushil, A. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar]
- Sadykov, V.; Mezentseva, N.; Alikina, G.; Bunina, R.; Pelipenko, V.; Lukashevich, A.; Vostrikov, Z.; Rogov, V.; Krieger, T.; Ishchenko, A.; et al. Nanocomposite Catalysts for Steam Reforming of Methane and Biofuels: Design and Performance. In Advances in Nanocomposites—Synthesis, Characterization and Industrial Applications; Reddy, B.S.R., Ed.; In Tech: Rijeka, Croatia, 2011; pp. 909–946. [Google Scholar]
- De Lima, S.M.; da Silva, A.M.; da Costa, L.O.O.; Graham, U.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Study of catalyst deactivation and reaction mechanism of steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Co/CeO2 catalyst. J. Catal. 2009, 268, 268–281. [Google Scholar] [CrossRef]
- Galetti, A.E.; Gomez, M.F.; Arrua, L.A.; Marchi, A.J.; Abello, M.C. Study of CuCoZnAl oxide as catalyst for the hydrogen production from ethanol reforming. Catal. Commun. 2008, 9, 1201–1208. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, L.; Qin, Y.N. Study on the carbon deposition in steam reforming of ethanol over Co/CeO2 catalyst. Chem. Eng. J. 2008, 145, 25–31. [Google Scholar] [CrossRef]
- Yu, S.W.; Huang, H.H.; Tang, C.W.; Wang, C.B. The effect of accessible oxygen over Co3O4-CeO2 catalysts on the steam reforming of ethanol. Int. J. Hydrogen Energy 2014, 39, 20700–20711. [Google Scholar] [CrossRef]
- Moon, D.J. Hydrogen production by catalytic reforming of liquid hydrocarbons. Catal. Surv. Asia 2011, 15, 25–36. [Google Scholar] [CrossRef]
- Finocchio, E.; Rossetti, I.; Ramis, G. Redox properties of Co- and Cu-based catalysts for the steam reforming of ethanol. Int. J. Hydrogen Energy 2013, 38, 3213–3225. [Google Scholar] [CrossRef]
- Da Silva, A.M.; de Souza, K.R.; Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. The effect of support reducibility on the stability of Co/CeO2 for the oxidative steam reforming of ethanol. Catal. Today 2011, 164, 234–239. [Google Scholar] [CrossRef]
- Mosqueda, B.; Toyir, J.; Kaddouri, A.; Gelin, P. Steam reforming of methane under water deficient conditions over gadolinium doped ceria. Appl. Catal. B 2009, 88, 361–367. [Google Scholar] [CrossRef]
- Al-Musa, A.A.; Ioakeimidis, Z.S.; Al-Saleh, M.S.; Al-Zahrany, A.; Marnellos, G.E.; Konsolakis, M. Steam reforming of iso-octane toward hydrogen production over mono- and bi-metallic Cu-Co/CeO2 catalysts: Structure-Activity Correlations. Int. J. Hydrogen Energy 2014, 39, 19541–19554. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Daniel, C.; van Veen, A.C.; Schuurman, Y.; Descorme, C.; Provendier, H.; Shen, W.; Mirodatos, C. Oxidative steam reforming of ethanol over Ir/CeO2 catalysts: A Structure Sensitivity Analysis. J. Catal. 2012, 286, 137–152. [Google Scholar] [CrossRef]
- Singhto, W.; Laosiripojana, N.; Assabumrungrat, S.; Charojrochkul, S. Steam reforming of bio-ethanol over Ni on Ce-ZrO2 support: Influence of Redox Properties on the Catalyst Reactivity. J. Sci. Technol. 2006, 28, 1251–1264. [Google Scholar]
- Laosiripojana, N.; Kiatkittipong, W.; Charojrochkul, S.; Assabumrungrat, S. Effects of support and co-fed elements on steam reforming of palm fatty acid distillate (PFAD) over Rh-based catalysts. Appl. Catal. A 2010, 383, 50–57. [Google Scholar] [CrossRef]
- Salazar-Villalpando, M.D.; Berry, D.A.; Cugini, A. Role of lattice oxygen in the partial oxidation of methane over Rh/zirconia-doped ceria. Isotopic studies. Int. J. Hydrogen Energy 2010, 35, 1998–2003. [Google Scholar] [CrossRef]
- Huang, T.J.; Wang, C.H. Roles of Surface and Bulk Lattice Oxygen in Forming CO2 and CO during Methane Reaction over Gadolinia-Doped Ceria. Catal. Lett. 2007, 118, 103–108. [Google Scholar] [CrossRef]















| Sample | SBET (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| CeO2 | 71.5 | 0.27 | 15.4 |
| 15 wt. % Co/CeO2 | 64.2 | 0.28 | 17.8 |
| 20 wt. % Co/CeO2 | 33.4 | 0.13 | 16.0 |
| 25 wt. % Co/CeO2 | 42.2 | 0.19 | 18.2 |
| 30 wt. % Co/CeO2 | 44.9 | 0.21 | 18.7 |
| 20 wt. % Ni/CeO2 | 57.6 | 0.54 | 37.7 |
| 20 wt. % Cu/CeO2 | 44.6 | 0.15 | 13.1 |
| 20 wt. % Fe/CeO2 | 57.0 | 0.26 | 18.3 |
| Samples | Phase Detected | Crystallite Size (nm) | Lattice |
|---|---|---|---|
| 15 wt.% Co/CeO2 | CeO2 | 11.5 | Cubic |
| Co3O4 | 30.8 | Cubic | |
| 20 wt.% Co/CeO2 | CeO2 | 10.2 | Cubic |
| Co3O4 | 37.7 | Cubic | |
| 25 wt.% Co/CeO2 | CeO2 | 13.6 | Cubic |
| Co3O4 | 32.3 | Cubic | |
| 30 wt.% Co/CeO2 | CeO2 | 10.4 | Cubic |
| Co3O4 | 37.7 | Cubic | |
| 20 wt.% Fe/CeO2 | CeO2 | 10.6 | Cubic |
| Fe2O3 | 34.1 | Rhombohedral | |
| 20 wt.% Ni/CeO2 | CeO2 | 11.2 | Cubic |
| NiO | 23.2 | Cubic | |
| 20 wt.% Cu/CeO2 | CeO2 | 9.3 | Cubic |
| CuO | 43.5 | Monoclinic |
| Sample | % | ||
|---|---|---|---|
| OI | OII | OIII | |
| Fe/CeO2 | 48 | 45 | 7 |
| Co/CeO2 | 70 | 24 | 6 |
| Ni/CeO2 | 46 | 54 | - |
| Cu/CeO2 | 38 | 55 | 7 |
| Samples | XPS a | Nominal b | ||||||
|---|---|---|---|---|---|---|---|---|
| M | Ce | O | M/Ce | M | Ce | O | M/Ce | |
| Fe/CeO2 | 7.7 | 29.6 | 62.7 | 0.26 | 15.6 | 20.3 | 64.1 | 0.77 |
| Co/CeO2 | 11.0 | 25.5 | 63.5 | 0.43 | 15.5 | 21.3 | 63.2 | 0.73 |
| Ni/CeO2 | 15.4 | 23.5 | 61.1 | 0.65 | 16.4 | 22.4 | 61.2 | 0.73 |
| Cu/CeO2 | 8.2 | 34.0 | 57.8 | 0.24 | 15.5 | 23.0 | 61.5 | 0.68 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G.E. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts 2016, 6, 39. https://doi.org/10.3390/catal6030039
Konsolakis M, Ioakimidis Z, Kraia T, Marnellos GE. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts. 2016; 6(3):39. https://doi.org/10.3390/catal6030039
Chicago/Turabian StyleKonsolakis, Michalis, Zisis Ioakimidis, Tzouliana Kraia, and George E. Marnellos. 2016. "Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship" Catalysts 6, no. 3: 39. https://doi.org/10.3390/catal6030039
APA StyleKonsolakis, M., Ioakimidis, Z., Kraia, T., & Marnellos, G. E. (2016). Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts, 6(3), 39. https://doi.org/10.3390/catal6030039