A Highly Efficient Dual Rotating Disks Photocatalytic Fuel Cell with Wedged Surface TiO2 Nanopore Anode and Hemoglobin Film Cathode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electron Transfer and Surface Reaction in a Typical RPFC System
2.2. Enhanced Light Absorbance using Wedged Surface TiO2 Nanopore Electrode
2.3. Degradation Performance of the RPFC System
3. Material and Methods
3.1. Materials and Reagents
3.2. Fabrication of TiO2/Ti Wedge-Surface Nanopore Rotating-Disk Anode
3.3. Immobilization of Hb on the Graphite Electrode
3.4. Characterization of the Electrodes
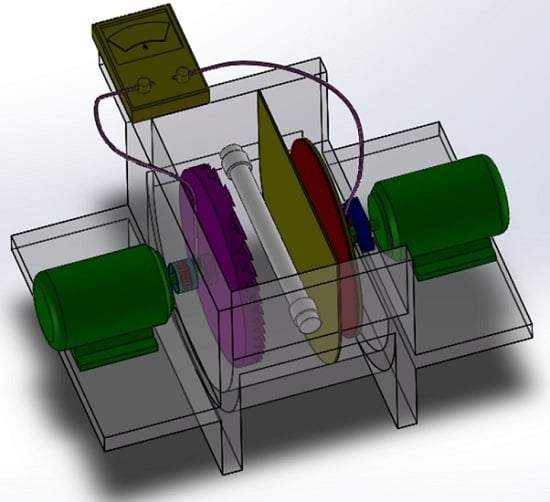
3.5. Dual Rotating-Disk Photocatalytic Fuel Cell
3.6. Performance of Organics Removal and Photocurrent Generation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Li, Z.S.; Kou, J.H.; Zou, Z.G. Mechanism investigation of visible light-induced degradation in a heterogeneous TiO2/Eosin Y/Rhodamine B system. Environ. Sci. Technol. 2009, 43, 8361–8366. [Google Scholar] [CrossRef] [PubMed]
- Dionysiou, D.D.; Balasubramanian, G.; Suidan, M.T.; Khodadoust, A.P.; Baudin, I.; Laîné, J.M. Rotating disk photocatalytic reactor: Development, characterization, and evaluation for the destruction of organic pollutants in water. Water Res. 2000, 34, 2927–2940. [Google Scholar] [CrossRef]
- Heylen, S.; Smet, S.; Laurier, K.G.M.; Hofkens, J.; Roeffaers, M.B.J.; Martens, J.A. Selective photocatalytic oxidation of gaseous ammonia to dinitrogen in a continuous flow reactor. Catal. Sci. Technol. 2012, 2, 1802–1805. [Google Scholar] [CrossRef]
- Cao, W.N.; Wang, F.; Wang, H.Y.; Chen, B.; Feng, K.; Tung, C.H.; Wu, L.Z. Photocatalytic hydrogen production from a simple water-soluble [FeFe]-hydrogenase model system. Chem. Commun. 2012, 48, 8081–8083. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Tsai, Y.C. An efficient 3C-silicon carbide/titania nanocomposite photoelectrode for dye-sensitized solar cell. Chem. Commun. 2012, 48, 6696–6698. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of Graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Matsui, T.; Ozaki, S.I.; Liong, E.; George, N.P., Jr.; Watanabe, Y. Effects of the location of distal histidine in the reaction of myoglobin with hydrogen peroxide. J. Biol. Chem. 1999, 274, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Mao, L.Y.; Cai, R.X. Stopped-flow spectrophotometric determination of hydrogen peroxide with hemoglobin as catalyst. Talanta. 2000, 51, 179–186. [Google Scholar] [CrossRef]
- Tang, T.T.; Hou, J.Y.; Ai, S.Y.; Qiu, Y.Y.; Ma, Q.; Han, R.X. Electroenzymatic oxidation of bisphenol A (BPA) based on the hemoglobin (Hb) film in a membraneless electrochemical reactor. J. Hazard. Mater. 2010, 181, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Gu, M.B.; Moon, S.H. Degradation of 2,4,6-trinitrotoluene by immobilized horseradish peroxidase and electrogenerated peroxide. Water Res. 2003, 37, 983–992. [Google Scholar]
- Kim, G.Y.; Moon, S.H. Degradation of pentachlorophenol by an electroenzymatic method using immobilized peroxidase enzyme. Korean J. Chem. Eng. 2005, 22, 52–60. [Google Scholar] [CrossRef]
- Cho, S.H.; Shim, J.; Yun, S.H.; Moon, S.H. Enzyme-catalyzed conversion of phenol by using immobilized horseradish peroxidase (HRP) in a membraneless electrochemical reactor. Appl. Catal. A. 2008, 337, 66–72. [Google Scholar] [CrossRef]
- Yao, Y.; Li, K.; Chen, S.; Jia, J.P.; Wang, Y.L.; Wang, H.W. Decolorization of Rhodamine B in a thin-film photoelectrocatalytic (PEC) reactor with slant-placed TiO2 nanotubes electrode. Chem. Eng. J. 2012, 187, 29–35. [Google Scholar] [CrossRef]
- Huo, Y.N.; Chen, X.F.; Zhang, J.; Pan, G.F.; Jia, J.P.; Li, H.X. Ordered macroporous Bi2O3/TiO2 film coated on a rotating disk with enhanced photocatalytic activity under visible irradiation. Appl. Catal. B 2014, 148–149, 550–556. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.B.; Tang, T.T.; Tang, Y.P.; Wang, Y.L.; Jia, J.P. Facile electrochemical polymerization of polypyrrole film applied as cathode material in dual rotating disk photo fuel cell. J. Power Sources 2016, 324, 368–377. [Google Scholar] [CrossRef]
- Tang, T.T.; Li, K.; Ying, D.W.; Sun, T.H.; Wang, Y.L.; Jia, J.P. High efficient aqueous-film rotating disk photocatalytic fuel cell (RDPFC) with triple functions: Cogeneration of hydrogen and electricity with dye degradation. Int. J. Hydrog. Energy 2014, 39, 10258–10266. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laîné, J.M. Oxidation of organic contaminants in a rotating disk photocatalytic reactor: reaction kinetics in the liquid phase and the role of mass transfer based on the dimensionless Damköhler number. Appl. Catal. B 2002, 38, 1–16. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Khodadoust, A.P.; Kern, A.M.; Suidan, M.T.; Baudin, I.; Laîné, J.M. Continuous-mode photocatalytic degradation of chlorinated phenols and pesticides in water using a bench-scale TiO2 rotating disk reactor. Appl. Catal. B 2000, 24, 139–155. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Burbano, A.A.; Suidan, M.T.; Baudin, I.; Laîné, J.M. Effect of oxygen in a thin-film rotating disk photocatalytic reactor. Environ. Sci. Technol. 2002, 36, 3834–3843. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Lianos, P. Production of electricity by photoelectrochemical oxidation of ethanol in a PhotoFuelCell. Appl. Catal. B 2010, 99, 307–313. [Google Scholar] [CrossRef]
- Xu, Y.L.; He, Y.; Cao, X.D.; Zhong, D.J.; Jia, J.P. TiO2/Ti rotating disk photoelectrocatalytic (PEC) reactor: A combination of highly effective thin-film PEC and conventional PEC processes on a single electrode. Environ. Sci. Technol. 2008, 42, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Wager, J.F. Transparent electronics: Schottky barrier and heterojunction considerations. Thin Solid Films 2008, 516, 1755–1764. [Google Scholar] [CrossRef]
- Xu, Y.L.; He, Y.; Jia, J.P.; Zhong, D.J.; Wang, Y.L. Cu-TiO2/Ti dual rotating disk photocatalytic (PC) reactor: Dual electrode degradation facilitated by spontaneous electron transfer. Environ. Sci. Technol. 2009, 43, 6289–6294. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z.Q. Freestanding TiO2 nanotube arrays with ultrahigh aspect ratio via electrochemical anodization. Chem. Mater. 2008, 20, 1257–1261. [Google Scholar] [CrossRef]
- Smith, Y.R.; Sarma, B.; Mohanty, S.K.; Misra, M. Light-assisted anodized TiO2 nanotube arrays. ACS Appl. Mater. Interfaces 2012, 4, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Liu, H.S. Development of an E-H2O2/TiO2 photoelectrocatalytic oxidation system for water and wastewater treatment. Environ. Sci. Technol. 2005, 39, 4614–4620. [Google Scholar] [CrossRef] [PubMed]
- Kar, T.; Munjal, M.L. Plane wave analysis of acoustic wedges using the boundary-condition-transfer algorithm. Appl. Acoust. 2006, 67, 901–917. [Google Scholar] [CrossRef]
- Sclafani, A.; Palmisano, L.; Schiavello, M. Influence of the preparation methods of titanium dioxide on the photocatalytic degradation of phenol in aqueous dispersion. J. Phys. Chem. 1990, 94, 829–832. [Google Scholar] [CrossRef]
- Aarthi, T.; Madras, G. Photocatalytic degradation of Rhodamine dyes with nano-TiO2. Ind. Eng. Chem. Res. 2007, 46, 7–14. [Google Scholar] [CrossRef]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; He, Y.; Li, K.; Ying, D.; Yao, Y.; Tang, T.; Wang, Y.; Jia, J. A Highly Efficient Dual Rotating Disks Photocatalytic Fuel Cell with Wedged Surface TiO2 Nanopore Anode and Hemoglobin Film Cathode. Catalysts 2016, 6, 114. https://doi.org/10.3390/catal6080114
Yang C, He Y, Li K, Ying D, Yao Y, Tang T, Wang Y, Jia J. A Highly Efficient Dual Rotating Disks Photocatalytic Fuel Cell with Wedged Surface TiO2 Nanopore Anode and Hemoglobin Film Cathode. Catalysts. 2016; 6(8):114. https://doi.org/10.3390/catal6080114
Chicago/Turabian StyleYang, Chen, Yi He, Kan Li, Diwen Ying, Ye Yao, Tiantian Tang, Yalin Wang, and Jinping Jia. 2016. "A Highly Efficient Dual Rotating Disks Photocatalytic Fuel Cell with Wedged Surface TiO2 Nanopore Anode and Hemoglobin Film Cathode" Catalysts 6, no. 8: 114. https://doi.org/10.3390/catal6080114
APA StyleYang, C., He, Y., Li, K., Ying, D., Yao, Y., Tang, T., Wang, Y., & Jia, J. (2016). A Highly Efficient Dual Rotating Disks Photocatalytic Fuel Cell with Wedged Surface TiO2 Nanopore Anode and Hemoglobin Film Cathode. Catalysts, 6(8), 114. https://doi.org/10.3390/catal6080114