Catalytic Isomerization of Dihydroxyacetone to Lactic Acid and Alkyl Lactates over Hierarchical Zeolites Containing Tin
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characteristics of Hierarchical Zeolites
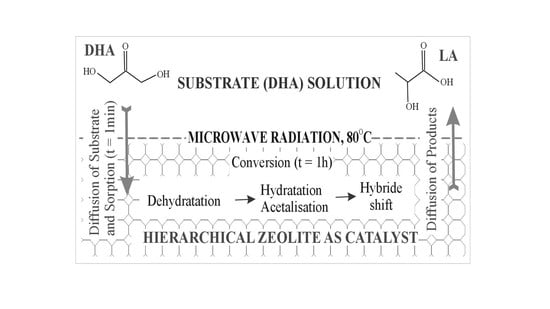
3.3. Catalytic Tests—DHA Isomerization Reaction to Alkyl Lactates and Lactic Acid
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malgras, V.; Ji, Q.; Kamachi, Y.; Mori, T.; Shieh, F.K.; Wu, K.C.V.; Ariga, K.; Yamauchi, Y. Templated Synthesis for Nanoarchitectured Porous Materials. Bull. Chem. Soc. Jpn. 2015, 88, 1171–1200. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Shieh, F.K.; Wang, S.C.; Yen, C.I.; Wu, C.C.; Dutta, S.; Chou, L.Y.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.W.; et al. Imparting functionality to biocatalysts via embedding enzymes into nanoporous materials by a de novo approach: Size-selective sheltering of catalase in metal–organic framework microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Shieh, F.K.; Hsiao, C.T.; Kao, H.M.; Sue, Y.C.; Lin, K.W.; Wu, C.C.; Chen, X.C.; Wan, L.; Hsu, M.H.; Hwu, J.R.; et al. Size-adjustable annular ring-functionalized mesoporous silica as effective and selective adsorbents for heavy metal ions. RSC Adv. 2013, 3, 25686–25689. [Google Scholar] [CrossRef]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with Mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Wei, Y.; Parmentier, T.E.; de Jong, K.P.; Zecevic, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef] [PubMed]
- Perez Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical Zeolites: enhanced Utilisation of Microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Gonche, A.; Schwieger, W. Catalytic test reactions for the evaluation of hierarchical zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef] [PubMed]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domınguez Gonzalez, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- García-Martínez, J.; Johnson, M.; Valla, J.; Li, K.; Ying, J.Y. Mesostructured zeolite Y—High hydrothermal stability and superior FCC catalytic performance. Catal. Sci. Technol. 2012, 2, 987–994. [Google Scholar] [CrossRef]
- Na, J.P.; Liu, G.Z.; Zhou, T.Y.; Ding, G.C.; Hu, S.L.; Wang, L. Synthesis and catalytic performance of ZSM-5/MCM-41 zeolites with varying Mesopore size by surfactant-directed recrystallization. Catal. Lett. 2013, 143, 267–275. [Google Scholar] [CrossRef]
- Guo, Y.P.; Wang, H.J.; Guo, Y.J.; Guo, L.H.; Chu, L.F.; Guo, C.X. Fabrication and characterization of hierarchical ZSM-5 zeolites by using organosilanes as additives. Chem. Eng. J. 2011, 166, 391–400. [Google Scholar] [CrossRef]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Gray, D.R. Morphologic classification of crenulation cleavage. J. Geol. 1977, 85, 229–235. [Google Scholar] [CrossRef]
- Müller, J.M.; Mesquita, G.C.; Franco, S.M.; Borges, L.D.; de Macedo, J.L.; Dias, J.A.; Dias, S.C.L. Solid-state dealumination of zeolites for use as catalysts in alcohol dehydration. Microporous Mesoporous Mater. 2015, 204, 50–57. [Google Scholar] [CrossRef]
- Bonilla, A.; Baudouin, D.; Pérez-Ramírez, J. Desilication of ferrierite zeolite for porosity generation and improved effectiveness in polyethylene pyrolysis. J. Catal. 2009, 265, 170–180. [Google Scholar] [CrossRef]
- Selvaraj, M.; Sinha, P.K. Highly selective and clean synthesis of nopol over well-ordered mesoporous tin silicate catalysts. New J. Chem. 2010, 34, 1921–1929. [Google Scholar] [CrossRef]
- Nowak, I.; Feliczak, A.; Nekoksova, I.; Cejka, J. Comparison of oxidation properties of Nb and Sn in mesoporous molecular sieves. Appl. Catal. A 2007, 321, 40–62. [Google Scholar] [CrossRef]
- Chaudhari, K.; Das, T.K.; Rajmohanan, P.R.; Lazar, K.; Sivasanker, S.; Chandwadkar, A.J. Synthesis, characterization, and catalytic properties of Mesoporous tin-containing analogs of MCM-41. J. Catal. 1999, 183, 281–291. [Google Scholar] [CrossRef]
- Casagrande, M.; Storaro, L.; Lenarda, M.; Gersich, J.; Stievano, L.; Wagner, F.E.; Montanari, T. Synthesis and structural characterization of ordered supermicroporous MSU type silica–tin molecular sieves. J. Mater. Chem. 2004, 14, 1010–1016. [Google Scholar] [CrossRef]
- Svelle, S.; Sommer, L.; Barbera, K.; Vennestrøm, P.N.R.; Olsbye, U.; Lillerud, K.P.; Bordiga, S.; Pan, Y.-H.; Beato, P. How defects and crystal morphology control the effects of desilication. Catal. Today 2011, 168, 38–47. [Google Scholar] [CrossRef]
- Lari, G.M.; García-Muelas, R.; Mondelli, C.; López, N.; Pérez-Ramírez, J. Glyceroloxidehydration to pyruvaldehyde over silver-based catalysts for improved lactic acid production. Green Chem. 2016, 18, 4682–4692. [Google Scholar] [CrossRef]
- Bordiga, S.; Lamberti, C.; Bonino, F.; Travertd, A.; Thibault-Starzyk, F. Probing zeolites by vibrational spectroscopies. Chem. Soc. Rev. 2015, 44, 7262–7341. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.P.; Aguado, J.; Morales, G.; Rodriguez, J.M.; Peral, A.; Thommes, M.; Epping, J.P.; Chmelka, B.F. Molecular and meso- and macroscopic properties of hierarchical Nanocrystalline ZSM-5 Zeolite Prepared by Seed Silanization. Chem. Mater. 2009, 21, 641–654. [Google Scholar] [CrossRef]
- Li, L.; Stroobants, C.; Lin, K.; Jacobs, P.A.; Sels, B.F.; Pescarmona, P.P. Selective conversion of trioses to lactates over Lewis acid heterogeneous catalysts. Green Chem. 2011, 13, 1175–1181. [Google Scholar] [CrossRef]
- De Clippel, F.M.; Dusselier, R.; Van Rompaey, P.; Vanelderen, J.; Dijkmans, E.; Makshina, L.; Giebeler, S.; Oswald, G.V.; Baron, J.F.M.; Denayer, P.P.; et al. Sels, fast and selective sugar conversion to Alkyl Lactate and lactic acid with Bifunctional carbon–silica catalysts. J. Am. Chem. Soc. 2012, 134, 10089–10101. [Google Scholar] [CrossRef] [PubMed]
- Pighin, E.; Díez, V.K.; Di Cosimo, J.I. Kinetic study of the ethyl lactate synthesis from triose sugars on Sn/Al2O3 catalysts. Catal. Today 2017, 289, 29–37. [Google Scholar] [CrossRef]
- Stiegman, A.E. Loss Mechanisms and Microwave-Specific Effects in Heterogeneous Catalysis, Microwaves in Catalysis: Methodology and Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 31–47. [Google Scholar]









| Materials | Content (%) | ||
|---|---|---|---|
| N | C | H | |
| HFAU commercial | 0.255 ± 0.005 | 0.151 ± 0.004 | 2.829 ± 0.005 |
| HFAU 0.5 h U 4 h mix | 0.146 ± 0.004 | 0.465 ± 0,002 | 2.606 ± 0.005 |
| SnFAU 0.5 h U 4 h mix | 0.199 ± 0.004 | 0.269± 0.005 | 2.762 ± 0.008 |
| NaBEA commercial | 0.273 ± 0.006 | 0.227 ± 0.004 | 1.403 ± 0.004 |
| NaBEA 0.5 h U 4 h mix | 0.255 ± 0.005 | 0.234 ± 0.005 | 0.711 ± 0.002 |
| SnBEA 0.5 h U 4 h mix | 0.199 ± 0.004 | 0.173 ± 0.006 | 1.235 ± 0.004 |
| Materials | Specific Surface Area (m2/g) | Pore Volume, BJH (cm3/g) | Average Submesopore * Size (nm) >2 nm | |||
|---|---|---|---|---|---|---|
| SBET | SLangmuir | Smicro t-Plot | Sext t-Plot | |||
| HFAU commercial | 718 | 972 | 946 | 26 | 0.05 | Absent |
| HFAU 0.5 h U 4 h mix | 791 | 1022 | 446 | 576 | 0.32 | 2.64 |
| SnFAU 0.5 h U 4 h mix | 946 | 1236 | 611 | 625 | 0.34 | 2.58 |
| NaBEA commercial | 567 | 715 | 450 | 265 | 0.09 | Absent |
| NaBEA 0.5 h U 4 h mix | 714 | 913 | 389 | 523 | 0.27 | 2.54 |
| SnBEA 0.5 h U 4 h mix | 653 | 895 | 485 | 410 | 0.32 | 2.56 |
| Entry | Catalyst | XDHA(%) | YML,EL,LA(%) | YPAL(%) | Time (h) |
|---|---|---|---|---|---|
| Catalytic conversion of DHA in Methanol—conventional method of heating (CM) 80 °C | |||||
| 1 | HFAU commercial | 43 | 38 | 5 | 5 |
| 2 | HFAU 0.5 h U 4 h mix | 62 | 45 | 15 | 5 |
| 3 | SnFAU 0.5 h U 4 h mix | 90 | 51 | 39 | 5 |
| 4 | NaBEA commercial | 39 | 35 | 4 | 5 |
| 5 | NaBEA 0.5 h U 4 h mix | 38 | 20 | 18 | 5 |
| 6 | SnBEA 0.5 h U 4 h mix | 84 | 59 | 25 | 5 |
| Catalytic conversion of DHA in Methanol—conventional method of heating (CM) 50 °C | |||||
| 7 | SnFAU 0.5 h U 4 h mix | 61 | 34 | 27 | 5 |
| 8 | SnBEA 0.5 h U 4 h mix | 57 | 32 | 25 | 5 |
| Catalytic conversion of DHA in Methanol—conventional method of heating (CM) 25 °C | |||||
| 9 | SnFAU 0.5 h U 4 h mix | 42 | 22 | 20 | 5 |
| 10 | SnBEA 0.5 h U 4 h mix | 27 | 7 | 20 | 5 |
| Catalytic conversion of DHA in Methanol—microwave radiation of heating (MR) 80 °C | |||||
| 11 | SnFAU 0.5 h U 4 h mix | 98 | 82 | 16 | 1 |
| 12 | SnBEA 0.5 h U 4 h mix | 90 | 76 | 14 | 1 |
| Catalytic conversion of DHA in Ethanol—conventional method of heating (CM) 80 °C | |||||
| 13 | SnFAU 0.5 h U 4 h mix | 93 | 73 | 20 | 5 |
| 14 | SnBEA 0.5 h U 4 h mix | 96 | 58 | 38 | 5 |
| Catalytic conversion of DHA in Ethanol—microwave radiation of heating (MR) 80 °C | |||||
| 15 | SnFAU 0.5 h U 4 h mix | 99 | 85 | 14 | 1 |
| 16 | SnBEA 0.5 h U 4 h mix | 98 | 72 | 26 | 1 |
| Catalytic conversion of DHA in Water—conventional method of heating (CM) 80 °C | |||||
| 17 | SnFAU 0.5 h U 4 h mix | 99 | 89 | 10 | 5 |
| 18 | SnFAU 0.5 h U 4 h mix—1st rec. | 99 | 88 | 11 | 5 |
| 19 | SnFAU 0.5 h U 4 h mix—2nd rec. | 98 | 87 | 12 | 5 |
| 20 | SnFAU 0.5 h U 4 h mix—3rd rec. | 97 | 86 | 12 | 5 |
| 22 | SnBEA 0.5 h U 4 h mix | 99 | 87 | 12 | 5 |
| Catalytic conversion of DHA in Water—microwave radiation of heating (MR) 80 °C | |||||
| 23 | SnFAU 0.5 h U 4 h mix | 100 | 92 | 8 | 1 |
| 24 | SnBEA 0.5 h U 4 h mix | 99 | 95 | 4 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliczak-Guzik, A.; Sprynskyy, M.; Nowak, I.; Buszewski, B. Catalytic Isomerization of Dihydroxyacetone to Lactic Acid and Alkyl Lactates over Hierarchical Zeolites Containing Tin. Catalysts 2018, 8, 31. https://doi.org/10.3390/catal8010031
Feliczak-Guzik A, Sprynskyy M, Nowak I, Buszewski B. Catalytic Isomerization of Dihydroxyacetone to Lactic Acid and Alkyl Lactates over Hierarchical Zeolites Containing Tin. Catalysts. 2018; 8(1):31. https://doi.org/10.3390/catal8010031
Chicago/Turabian StyleFeliczak-Guzik, Agnieszka, Myroslav Sprynskyy, Izabela Nowak, and Bogusław Buszewski. 2018. "Catalytic Isomerization of Dihydroxyacetone to Lactic Acid and Alkyl Lactates over Hierarchical Zeolites Containing Tin" Catalysts 8, no. 1: 31. https://doi.org/10.3390/catal8010031
APA StyleFeliczak-Guzik, A., Sprynskyy, M., Nowak, I., & Buszewski, B. (2018). Catalytic Isomerization of Dihydroxyacetone to Lactic Acid and Alkyl Lactates over Hierarchical Zeolites Containing Tin. Catalysts, 8(1), 31. https://doi.org/10.3390/catal8010031