Chloroperoxidase-Mediated Halogenation of Selected Pharmaceutical Micropollutants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Product Identification
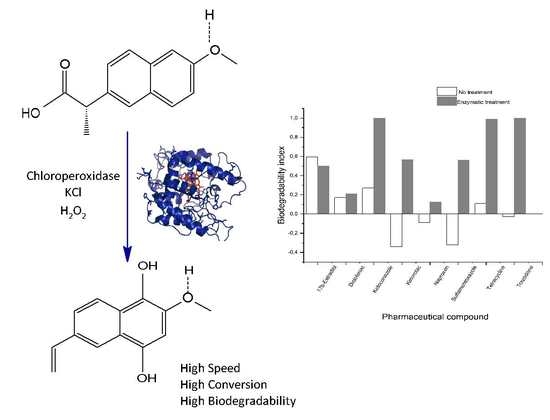
2.2. Studies of the Biodegradability of the PhCs and the Enzymatic Reaction Products
2.3. Application of the Enzymatic Treatment in Simulated Treated Wastewater
3. Materials and Methods
3.1. Chemicals
3.2. Enzymatic Activity in Model Systems
3.3. Kinetic Constants
3.4. Enzyme Immobilization
3.5. HPLC Analysis
3.6. Product Extraction
3.7. Mass Spectrometry Analysis
3.8. Enzymatic Oxidation of PhCs in Simulated Treated Water
3.9. Biodegradability Determination
3.10. Raman Measurements
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Pal, A.; Gin, K.Y.; Lin, A.Y.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Méndez, E.; González-Fuentes, M.A.; Rebollar-Perez, G.; Méndez-Albores, A.; Torres, E. Emerging pollutant treatments in wastewater: Cases of antibiotics and hormones. J. Environ. Sci. Health Part A 2017, 52, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Ncibi, M.C.; Mahjoub, B.; Mahjoub, O.; Sillanpää, M. Remediation of Emerging Pollutants in Contaminated Wastewater and Aquatic Environments: Biomass-Based Technologies. CLEAN—Soil Air Water 2017, 45. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.H.; Lim, T.T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Eibes, G.; Debernardi, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Oxidation of pharmaceutically active compounds by a ligninolytic fungal peroxidase. Biodegradation 2011, 22, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Majeau, J.A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef] [PubMed]
- Taboada-Puig, R.; Eibes, G.; Lloret, L.; Lú-Chau, T.A.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Fostering the action of versatile peroxidase as a highly efficient biocatalyst for the removal of endocrine disrupting compounds. New Biotechnol. 2016, 33, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duarte, C.; Viana, M.T.; Vazquez-Duhalt, R. Laccase-Mediated Transformations of Endocrine Disrupting Chemicals Abolish Binding Affinities to Estrogen Receptors and Their Estrogenic Activity in Zebrafish. Appl. Biochem. Biotechnol. 2012, 168, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duarte, C.; Vazquez-Duhalt, R. Applications and Prospective of Peroxidase Biocatalysis in the Environmental Field. In Biocatalysis Based on Heme Peroxidases; Torres, E., Ayala, M., Eds.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Garcia-Morales, R.; Rodríguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.; Torres, E.; Parra, R.; Cárdenas-Chávez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of Endocrine-Disrupting Compounds in Groundwater: Bisphenol A, Nonylphenol, Ethynylestradiol and Triclosan by a Laccase Cocktail from Pycnoporus sanguineus CS43. Water Air Soil Pollut. 2015, 226, 251. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Bustos-Jaimes, I.; le Borgne, S. Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl. Catal. B Environ. 2003, 46, 1–15. [Google Scholar] [CrossRef]
- Murugesan, K.; Chang, Y.Y.; Kim, Y.M.; Jeon, J.R.; Kim, E.J.; Chang, Y.S. Enhanced transformation of triclosan by laccase in the presence of redox mediators. Water Res. 2010, 44, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Lloret, L.; Eibes, G.; Lú-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalyzed degradation of anti-inflammatories and estrogens. Biochem. Eng. J. 2010, 51, 124–131. [Google Scholar] [CrossRef]
- Wen, X.; Jia, Y.; Li, J. Degradation of tetracycline and oxytetracycline by crude lignin peroxidase prepared from Phanerochaete chrysosporium—A white rot fungus. Chemosphere 2009, 75, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hirai, H.; Murata, H.; Nishida, T. Removal of estrogenic activities of 17β-estradiol and ethinylestradiol by ligninolytic enzymes from white rot fungi. Water Res. 2003, 37, 1972–1975. [Google Scholar] [CrossRef]
- Montiel, C.; Terrés, E.; Domínguez, J.M.; Aburto, J. Immobilization of chloroperoxidase on silica-based materials for 4,6-dimethyl dibenzothiophene oxidation. J. Mol. Catal. B Enzym. 2007, 48, 90–98. [Google Scholar] [CrossRef]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Catalytic activity in organic solvents and stability of immobilized enzymes depend on the pore size and surface characteristics of mesoporous silica. Chem. Mater. 2000, 12, 3301–3305. [Google Scholar] [CrossRef]
- Pešić, M.; López, C.; Álvaro, G.; López-Santín, J. A novel immobilized chloroperoxidase biocatalyst with improved stability for the oxidation of amino alcohols to amino aldehydes. J. Mol. Catal. B Enzym. 2012, 84, 144–151. [Google Scholar] [CrossRef]
- Hernandez, J.; Robledo, N.R.; Velasco, L.; Quintero, R.; Pickard, M.A.; Vazquez-Duhalt, R. Chloroperoxidase-Mediated Oxidation of Organophosphorus Pesticides. Pestic. Biochem. Physiol. 1998, 61, 87–94. [Google Scholar] [CrossRef]
- Vázquez-Duhalt, R.; Ayala, M.; Márquez-Rocha, F.J. Biocatalytic chlorination of aromatic hydrocarbons by chloroperoxidase of Caldariomyces fumago. Phytochemistry 2001, 58, 929–933. [Google Scholar] [CrossRef]
- Guerrero, E.; Aburto, P.; Terrés, E.; Villegas, O.; González, E.; Zayas, T.; Hernández, F.; Torres, E. Improvement of catalytic efficiency of chloroperoxidase by its covalent immobilization on SBA-15 for azo dye oxidation. J. Porous Mater. 2013, 20, 387–396. [Google Scholar] [CrossRef]
- Ayala, M.; Robledo, N.R.; Lopez-Munguia, A.; Vazquez-Duhalt, R. Substrate Specificity and Ionization Potential in Chloroperoxidase-Catalyzed Oxidation of Diesel Fuel. Environ. Sci. Technol. 2000, 34, 2804–2809. [Google Scholar] [CrossRef]
- Correa-Basurto, J.; Aburto, J.; Trujillo-Ferrara, J.; Torres, E. Ligand recognition by chloroperoxidase using molecular interaction fields and quantum chemistry calculations. Mol. Simul. 2007, 33, 649–654. [Google Scholar] [CrossRef]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.M.; Hickey, R.; Hsu, T.; Betancourt Román, C.M.; Chen, J.; Schwager, R.; Kline, J.; Brown, G.Z.; Halden, R.U.; Huttenhower, C.; et al. Antimicrobial Chemicals Are Associated with Elevated Antibiotic Resistance Genes in the Indoor Dust Microbiome. Environ. Sci. Technol. 2016, 50, 9807–9815. [Google Scholar] [CrossRef] [PubMed]
- Van Breda, L.K.; Ward, M.P. Evidence of antimicrobial and disinfectant resistance in a remote, isolated wild pig population. Prev. Vet. Med. 2017, 147, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Bácsi, I.; Viktória, B.; Kókai, Z.; Gonda, S.; Novák, Z.; Nagy, S.A.; Vasas, G. Effects of non-steroidal anti-inflammatory drugs on cyanobacteria and algae in laboratory strains and in natural algal assemblages. Environ. Pollut. 2016, 212, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Redshaw, C.H. Evaluation of biological endpoints in crop plants after exposure to non-steroidal anti-inflammatory drugs (NSAIDs): Implications for phytotoxicological assessment of novel contaminants. Ecotoxicol. Environ. Saf. 2015, 112, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Longoria, A.; Tinoco, R.; Vázquez-Duhalt, R. Chloroperoxidase-mediated transformation of highly halogenated monoaromatic compounds. Chemosphere 2008, 72, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Eisenthal, R.; Danson, M.J.; Hough, D.W. Catalytic efficiency and kcat/KM: A useful comparator? Trends Biotechnol. 2007, 25, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Q.; Li, H.; Gao, X.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y.; Wang, X. Bioconversion of non-steroidal anti-inflammatory drugs diclofenac and naproxen by chloroperoxidase. Biochem. Eng. J. 2017, 120, 7–16. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Combination of enzymatic degradation by chloroperoxidase with activated sludge treatment to remove sulfamethoxazole: Performance, and eco-toxicity assessment. J. Chem. Technol. Biotechnol. 2016, 91, 2802–2809. [Google Scholar] [CrossRef]
- Salcedo, K.; Torres-Ramírez, E.; Haces, I.; Ayala, M. Halogenation of β-estradiol by a rationally designed mesoporous biocatalyst based on chloroperoxidase. Biocatalysis 2015, 1, 33–43. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Shoemaker, J.A. Effects of Chlorination on the Persistence of Pharmaceuticals in the Environment. Bull. Environ. Contam. Toxicol. 2005, 74, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dodd, M.C.; Huang, C.H. Transformation of the Antibacterial Agent Sulfamethoxazole in Reactions with Chlorine: Kinetics, Mechanisms, and Pathways. Environ. Sci. Technol. 2004, 38, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Bedner, M.; MacCrehan, W.A. Transformation of Acetaminophen by Chlorination Produces the Toxicants 1,4-Benzoquinone and N-Acetyl-p-benzoquinone Imine. Environ. Sci. Technol. 2005, 40, 516–522. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Liu, H.; Burdette, J.E.; Li, Y.; Overk, C.R.; Pisha, E.; Yao, J.; van Breemen, R.B.; Swanson, S.M.; et al. Effect of Halogenated Substituents on the Metabolism and Estrogenic Effects of the Equine Estrogen, Equilenin. Chem. Res. Toxicol. 2003, 16, 741–749. [Google Scholar] [CrossRef] [PubMed]
- The Organisation for Economic Co-Operation and Development (OECD). Test No. 301: Ready Biodegradability; OECD Publishing: Paris, France, 1992. [Google Scholar]
- Liu, C.; Thormann, E.; Claesson, P.M.; Tyrode, E. Surface Grafted Chitosan Gels. Part II. Gel Formation and Characterization. Langmuir 2014, 30, 8878–8888. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–806. [Google Scholar] [PubMed]
- Praveena, S.D.; Ravindrachary, V.; Bhajantri, R.F.; Ismayil. Free volume-related microstructural properties of lithium perchlorate/sodium alginate polymer composites. Polym. Compos. 2014, 35, 1267–1274. [Google Scholar] [CrossRef]
- Boujday, S.; Chapelle, M.L.; Srajer, J.; Knoll, W. Enhanced Vibrational Spectroscopies as Tools for Small Molecule Biosensing. Sensors 2015, 15, 21239–21264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez-Duhalt, R.; Tinoco, R.; D’Antonio, P.; Topoleski, L.T.; Payne, G.F. Enzyme Conjugation to the Polysaccharide Chitosan: Smart Biocatalysts and Biocatalytic Hydrogels. Bioconjug. Chem. 2001, 12, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Aburto, J.; Ayala, M.; Bustos-Jaimes, I.; Montiel, C.; Terrés, E.; Dominguez, J.M.; Torres, E. Stability and catalytic properties of chloroperoxidase immobilized on SBA-16 mesoporous materials. Microporous Mesoporous Mater. 2005, 83, 193–200. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shiller, A.M. Distribution of hydrogen peroxide in the northwest Pacific Ocean. Geochem. Geophys. Geosyst. 2005, 6. [Google Scholar] [CrossRef]
- Chiu, S.H.; Chung, T.W.; Giridhar, R.; Wu, W.T. Immobilization of β-cyclodextrin in chitosan beads for separation of cholesterol from egg yolk. Food Res. Int. 2004, 37, 217–223. [Google Scholar] [CrossRef]







| Compound | λHPLC (nm) | kcat (s−1) | KmH2O2 (mM) | kcat/KmH2O2 (s−1·mM−1) |
|---|---|---|---|---|
| Naproxen | 260 | 267 | 0.10 | 2666 |
| Diclofenac | 220 | 143 | 0.06 | 2383 |
| Sulfamethoxazole | 270 | 179 | 0.13 | 1379 |
| 17β-Estradiol | 280 | 136 | 0.12 | 1133 |
| Ketoconazole | 240 | 141 | 0.18 | 783 |
| Tetracycline | 350 | 100 | 0.27 | 370 |
| Ketorolac | 315 | 39 | 0.11 | 359 |
| Trazodone | 280 | 19 | 0.09 | 211 |
| PhCs | Adsorption (%) | Conversion (%) | Total Removal (%) |
|---|---|---|---|
| 17β-Estradiol | 12.27 ± 1.97 | 70.40 ± 0.66 | 82.67 |
| Diclofenac | 10.88 ±1.58 | 83.99 ± 0.81 | 94.87 |
| Ketoconazole | 23.04 ± 2.27 | 75.96 ± 0.45 | 99.00 |
| Ketorolac | 3.27 ± 1.85 | 92.98 ± 0.26 | 96.98 |
| Naproxen | 0.86 ± 2.22 | 84.78 ± 0.49 | 85.64 |
| Sulfamethoxazole | 31.85 ± 2.15 | 64.95 ± 0.54 | 96.80 |
| Tetracycline | 10.34 ± 0.79 | 88.79 ± 0.05 | 99.13 |
| Trazodone | 10.43 ± 2.39 | 14.76 ± 0.36 | 25.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Zamora, J.L.; León-Aguirre, K.; Quiroz-Morales, R.; Parra-Saldívar, R.; Gómez-Patiño, M.B.; Arrieta-Baez, D.; Rebollar-Pérez, G.; Torres, E. Chloroperoxidase-Mediated Halogenation of Selected Pharmaceutical Micropollutants. Catalysts 2018, 8, 32. https://doi.org/10.3390/catal8010032
García-Zamora JL, León-Aguirre K, Quiroz-Morales R, Parra-Saldívar R, Gómez-Patiño MB, Arrieta-Baez D, Rebollar-Pérez G, Torres E. Chloroperoxidase-Mediated Halogenation of Selected Pharmaceutical Micropollutants. Catalysts. 2018; 8(1):32. https://doi.org/10.3390/catal8010032
Chicago/Turabian StyleGarcía-Zamora, José Luis, Karina León-Aguirre, René Quiroz-Morales, Roberto Parra-Saldívar, Mayra Beatriz Gómez-Patiño, Daniel Arrieta-Baez, Georgette Rebollar-Pérez, and Eduardo Torres. 2018. "Chloroperoxidase-Mediated Halogenation of Selected Pharmaceutical Micropollutants" Catalysts 8, no. 1: 32. https://doi.org/10.3390/catal8010032
APA StyleGarcía-Zamora, J. L., León-Aguirre, K., Quiroz-Morales, R., Parra-Saldívar, R., Gómez-Patiño, M. B., Arrieta-Baez, D., Rebollar-Pérez, G., & Torres, E. (2018). Chloroperoxidase-Mediated Halogenation of Selected Pharmaceutical Micropollutants. Catalysts, 8(1), 32. https://doi.org/10.3390/catal8010032