Highly Efficient Synthesis of 2,5-Dihydroxypyridine using Pseudomonas sp. ZZ-5 Nicotine Hydroxylase Immobilized on Immobead 150
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immobilization of Purified HSPHZZ on Immobead 150
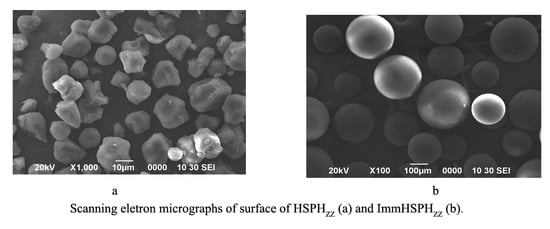
2.2. Scanning Electron Microscopy (SEM) Analysis of HSPHZZ and ImmHSPHZZ
2.3. Effect of pH on the Activity of HSPHZZ and ImmHSPHZZ
2.4. Effect of Temperature on the Activity of HSPHZZ and ImmHSPHZZ
2.5. Effect of Enzymatic Concentration on the Activity of ImmHSPHZZ
2.6. Effect of HSP Concentration on the Activity of ImmHSPHZZ
2.7. 2,5-DHP Production from HSP by ImmHSPHZZ under Optimum Conditions
2.8. Stability of Storage and Reusability
3. Materials and Methods
3.1. Materials
3.2. Multipoint Immobilization of HSPHZZ on Immobead 150
3.3. Characterization of HSPHZZ and ImmHSPHZZ
3.4. Stability of ImmHSPHZZ
3.5. SEM Assay of ImmHSPHZZ
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, R. Microbiology and biochemistry of nicotine degradation. Appl. Microbiol. Biotechnol. 2006, 69, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Novotny, T.E.; Zhao, F. Consumption and production waste: Another externality of tobacco use. Tob. Control 1999, 8, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Z.; Wang, L.J.; Wang, W.W.; Yu, H.; Zhang, K.Z.; Yao, Y.X.; Xu, P. Systematic unraveling of the unsolved pathway of nicotine degradation in Pseudomonas. PLoS Genet. 2013, 9, e1003923. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.H.; Zhu, C.J.; Shu, M.; Sun, K.D.; Zhao, L.; Wang, C.; Ye, Z.J.; Chen, J.M. Degradation of nicotine in tobacco waste extract by newly isolated Pseudomonas sp. ZUTSKD. Bioresour. Technol. 2010, 101, 6935–6941. [Google Scholar] [CrossRef] [PubMed]
- Ruan, A.D.; Min, H.; Zhu, W. Studies on biodegradation of nicotine by Arthrobacter sp strain HF-2. J. Environ. Sci. Health B 2006, 41, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Z.; Yang, G.Q.; Min, H.; Lv, Z.M. A novel nicotine catabolic plasmid pMH1 in Pseudomonas sp strain HF-1. Can. J. Microbiol. 2009, 55, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.G.; Ma, Y.; Wen, Y.Z.; Chen, L.S.; Wu, L.F.; Liu, W.P. Functional identification of two novel genes from Pseudomonas sp. strain HZN6 involved in the catabolism of nicotine. Appl. Environ. Microbiol. 2012, 78, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, H.; Xie, K.; Xu, P. Identification of nicotine biotransformation intermediates by Agrobacterium tumefaciens strain S33 suggests a novel nicotine degradation pathway. Appl. Microbiol. Biotechnol. 2012, 95, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Lu, L.L.; Gu, G.F.; Xiao, M. A novel pathway for nicotine degradation by Aspergillus oryzae 112822 isolated from tobacco leaves. Res. Microbiol. 2010, 161, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Z.; Yang, G.Q.; Wang, X.; Yao, Y.L.; Min, H.; Lu, Z.M. Nicotine degradation by two novel bacterial isolates of Acinetobacter sp TW and Sphingomonas sp TY and their responses in the presence of neonicotinoid insecticides. World J. Microb. Microbiol. 2011, 27, 1633–1640. [Google Scholar] [CrossRef]
- Masai, E.; Harada, K.; Peng, X.; Kitayama, H.; Katayama, Y.; Fukuda, M. Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl. Environ. Microbiol. 2002, 68, 4416–4424. [Google Scholar] [CrossRef] [PubMed]
- Plaggenborg, R.; Overhage, J.; Loos, A.; Archer, J.A.; Lessard, P.; Sinskey, A.J.; Steinbuchel, A.; Priefert, H. Potential of Rhodococcus strains for biotechnological vanillin production from ferulic acid and eugenol. Appl. Environ. Microbiol. 2006, 72, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Kachalova, G.; Decker, K.; Holt, A.; Bartunik, H.D. Crystallographic snapshots of the complete reaction cycle of nicotine degradation by an amine oxidase of the monoamine oxidase (MAO) family. Proc. Natl. Acad. Sci. USA 2011, 108, 4800–4805. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Xu, P.; Tang, H.Z.; Meng, J.; Liu, X.L.; Qing, C. “Green” route to 6-hydroxy-3-succinoyl-pyridine from (S)-nicotine of tobacco waste by whole cells of a Pseudomonas sp. Environ. Sci. Technol. 2005, 39, 6877–6880. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Warloe, T.; Berg, K.; Moan, J.; Kongshaug, M.; Giercksky, K.E.; Nesland, J.M. 5-Aminolevulinic acid-based photodynamic therapy: Clinical research and future challenges. Cancer 1997, 79, 2282–2308. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Wang, Y.P.; Wang, H.; Zhou, L.Y.; Gao, J.; Zhang, Y.F.; Zhang, X.; Wang, X.M.; Li, J. Facile immobilization of enzyme on three dimensionally ordered macroporous silica via a biomimetic coating. New J. Chem. 2015, 39, 978–984. [Google Scholar] [CrossRef]
- Alagoz, D.; Tukel, S.S.; Yildirim, D. Purification, immobilization and characterization of (R)-hydroxynitrile lyase from Prunus amygdalus turcomanica seeds and their applicability for synthesis of enantiopure cyanohydrins. J. Mol. Catal. B Enzym. 2014, 101, 40–46. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderrhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pelt, S.V. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nie, T.T.; Shao, L.L.; Cui, Z. Comparison of physical and covalent immobilization of lipase from Candida antarctica on polyamine microspheres of alkylamine matrix. Biocatal. Biotransform. 2014, 32, 314–326. [Google Scholar] [CrossRef]
- Knezevic, Z.; Milosavic, N.; Bezbradica, D.; Jakovljevic, Z.; Prodanovic, R. Immobilization of lipase from Candida rugosa on Eupergit® C supports by covalent attachment. Biochem. Eng. J. 2006, 30, 269–278. [Google Scholar] [CrossRef]
- Wei, T.; Zang, J.; Zheng, Y.D.; Tang, H.Z.; Huang, S.; Mao, D.B. Characterization of a novel nicotine hydroxylase from Pseudomonas sp. ZZ-5 that catalyzes the conversion of 6-Hydroxy-3-Succinoylpyridine into 2,5-Dihydroxypyridine. Catalysts 2017, 7, 257. [Google Scholar] [CrossRef]
- Carla, R.M.; Carolina, B.; Poppe, J.K.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A.Z. Synthesis of butyl butyrate in batch and continuous enzymatic reactors using Thermomyces lanuginosus lipase immobilized in Immobead 150. J. Mol. Catal. B Enzym. 2016, 127, 67–75. [Google Scholar]
- Elnashar, M.M.; Hassan, M.E.; Ghada, E.A. Grafted carrageenan gel disks and beads with polyethylenimine and glutaraldehyde for covalent immobilization of Penicillin G acylase. J. Colloid Interface Sci. 2013, 393, 27–33. [Google Scholar] [CrossRef]
- Yuan, Y.; Luan, X.N.; Rana, X.K.; Hassan, M.E.; Dou, D.Q. Covalent immobilization of cellulase in application of biotransformation of ginsenoside Rb1. J. Mol. Catal. B Enzym. 2016, 133, S525–S532. [Google Scholar] [CrossRef]
- Chen, S.H.; Yen, Y.H.; Wang, C.L.; Wang, S.L. Reversible immobilization of lysozyme via coupling to reversibly soluble polymer. Enzym. Microb. Technol. 2003, 33, 643–649. [Google Scholar] [CrossRef]
- Wei, T.; Yu, X.; Wang, Y.Y.; Zhu, Y.H.; Du, C.C.; Jia, C.X.; Mao, D.B. Purification and evaluation of the enzymatic properties of a novel fructosyltransferase from Aspergillus oryzae: A potential biocatalyst for the synthesis of sucrose 6-acetate. Biotechnol. Lett. 2014, 36, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bai, S.; Ren, M.; Sun, Y. Optimization of lipase-catalyzed enantioselective esterification of (±)-menthol in ionic liquid. Food Chem. 2008, 109, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Feng, S.X.; Mao, D.B.; Yu, X.; Du, C.C.; Wang, X.H. Characterization of a new thermophilic and acid tolerant esterase from Thermotoga maritima capable of hydrolytic resolution of racemic ketoprofen ethyl ester. J. Mol. Catal. B Enzym. 2013, 85, 23–30. [Google Scholar]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, G. Lipase immobilization on glutaraldehyde-activated nanofibrous membranes for improved enzyme stabilities and activities. React. Funct. Polym. 2012, 72, 839–845. [Google Scholar] [CrossRef]
- Kuo, C.H.; Liu, Y.C.; Chang, C.M.J.; Chen, J.H.; Chang, C.; Shieh, C.J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr. Polym. 2012, 87, 2538–2545. [Google Scholar] [CrossRef]
- Milasinovic, N.; Knezevic-Jugovic, Z.; Jakovljevic, Z.; Filipovic, J.; Krusic, M.K. Synthesis of n-amyl isobutyrate catalyzed by Candida rugosa lipase immobilized into poly(N-isopropylacrylamide-co-itaconic acid) hydrogels. Chem. Eng. J. 2012, 181–182, 614–623. [Google Scholar] [CrossRef]
- Madalozzo, A.D.; Martini, V.P.; Kuniyoshi, K.K.; de Souza, E.M.; Pedrosa, F.O.; Glogauer, A.; Zanin, G.M.; Mitchell, D.A.; Krieger, N. Immobilization of LipC12, a new lipase obtained by metagenomics, and its application in the synthesis of biodiesel esters. J. Mol. Catal. B Enzym. 2015, 116, 45–51. [Google Scholar] [CrossRef]








| - | Protein Loading (mg g-1) | Immobilization Efficiency (IE) (%) | Retention of Activity (R) (%) |
|---|---|---|---|
| Immobead 150 | 5 | 100 | 78 |
| 10 | 100 | 85 | |
| - | 15 | 100 | 95 |
| - | 20 | 100 | 75 |
| - | 25 | 90 | 51 |
| - | 30 | 67 | 45 |
| Protein | Substrate | kcat (s−1) | Km (mM) | kcat/Km (s−1 mM−1) |
|---|---|---|---|---|
| HSPHZZ | HSP | 1.9 ± 0.3 | 0.18 ± 0.02 | 10.6 ± 2.1 |
| - | NADH | 1.5 ± 0.4 | 0.18 ± 0.04 | 8.3 ± 1.9 |
| ImmHSPHZZ | HSP | 4.8 ± 0.4 | 0.20 ± 0.02 | 24.0 ± 2.1 |
| - | NADH | 5.2 ± 0.2 | 0.22 ± 0.05 | 23.6 ± 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Zheng, Y.; Tang, H.; Long, Z.; Li, J.; Zhang, Z.; Liu, S.; Mao, D.; Wei, T. Highly Efficient Synthesis of 2,5-Dihydroxypyridine using Pseudomonas sp. ZZ-5 Nicotine Hydroxylase Immobilized on Immobead 150. Catalysts 2018, 8, 548. https://doi.org/10.3390/catal8110548
Dong C, Zheng Y, Tang H, Long Z, Li J, Zhang Z, Liu S, Mao D, Wei T. Highly Efficient Synthesis of 2,5-Dihydroxypyridine using Pseudomonas sp. ZZ-5 Nicotine Hydroxylase Immobilized on Immobead 150. Catalysts. 2018; 8(11):548. https://doi.org/10.3390/catal8110548
Chicago/Turabian StyleDong, Caiwen, Yadong Zheng, Hongzhi Tang, Zhangde Long, Jigang Li, Zhiping Zhang, Sumeng Liu, Duobin Mao, and Tao Wei. 2018. "Highly Efficient Synthesis of 2,5-Dihydroxypyridine using Pseudomonas sp. ZZ-5 Nicotine Hydroxylase Immobilized on Immobead 150" Catalysts 8, no. 11: 548. https://doi.org/10.3390/catal8110548
APA StyleDong, C., Zheng, Y., Tang, H., Long, Z., Li, J., Zhang, Z., Liu, S., Mao, D., & Wei, T. (2018). Highly Efficient Synthesis of 2,5-Dihydroxypyridine using Pseudomonas sp. ZZ-5 Nicotine Hydroxylase Immobilized on Immobead 150. Catalysts, 8(11), 548. https://doi.org/10.3390/catal8110548