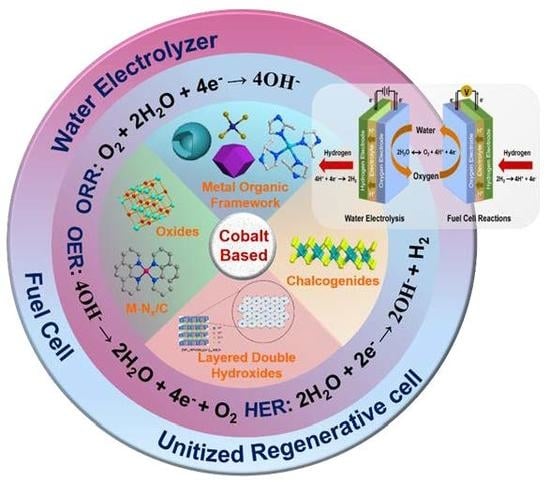
Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Electrocatalysts for Oxygen Reduction Reaction (ORR)
2.1. Mechanism of ORR
2.2. Oxygen Reduction on Cobalt Chalcogenides Catalysts
2.2.1. Bond Ionicity or Covalency of S, Se, Te
2.2.2. Crystal Structure and Particle Size Effect
2.2.3. Synthesis and Support Effect
2.3. Oxygen Reduction on Metal–Organic Frameworks (MOFs) Catalysts
2.4. Oxygen Reduction on Cobalt Oxide Catalysts
2.4.1. Nanostructure
2.4.2. Particle Size Effect and Chemical Composition
2.4.3. Support Effect
2.5. Oxygen Reduction on Cobalt-Based Layered Double-Hydroxides Catalysts
Synthesis Strategy
2.6. Oxygen Reduction on Co–Nx/C Catalysts
2.6.1. Co–Nx Active center
2.6.2. Co–NxSynthesis Strategy
3. Electrocatalysts for Oxygen Evolution Reaction (OER)
3.1. Mechanistic Approach of OER
3.2. Oxygen Evolution on Cobalt Chalcogenides Catalysts
3.2.1. Synthesis Strategy
3.2.2. Support Effect
3.3. Oxygen Evolution on Cobalt Oxides Catalysts
3.3.1. Mechanism of Cobalt Oxides
3.3.2. Chemical Composition
3.3.3. Synthesis Strategy
3.4. Oxygen Evolution on Cobalt-Based Layered Double-Hydroxides Catalysts
3.5. Cobalt-Based Bifunctional Catalysts in Assembled Unitized Regenerative Fuel Cells
4. Electrocatalysts for Hydrogen Evolution Reaction (HER)
4.1. Mechanism of HER
- A hydrogen atom adsorption, which is the result of the combination of a proton and an electron on the electrode surface (proton discharge) is the Volmer reaction:∗ + H+ + e− ⇌ ∗Had
- The adsorbed hydrogen atom interacting with a proton and an electron leads to an electrochemical desorption. This reaction is the Heyrovsky reaction:∗Had + H+ + e− ⇌ H2 + ∗
- The coupling of the two adsorbed hydrogen atoms leads to a dissociative desorption of hydrogen, the Tafel reaction:
- For the Heyrovsky reaction, the adsorbed hydrogen atom combines with molecular water and an electron, allowing the electrochemical desorption of hydrogen:
- The Tafel reaction is similar to that of the acidic medium.
4.2. Hydrogen Evolution on Cobalt Chalcogenides Catalysts
4.2.1. Synthesis Strategy
4.2.2. Crystal Structure and Nanostrcuture
4.3. Hydrogen Evolution on MOFs Catalysts
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, S.; Shao, Y.Y.; Liu, J.; Wang, Y. Electrocatalysts for Water Electrolyzers and Reversible Fuel Cells: Status and Perspective. Energy Environ. Sci. 2012, 5, 9331–9344. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells –Fundamentals and Applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Shao, M.H.; Chang, Q.W.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.M.; Xue, Y.H.; Qu, L.T.; Choi, H.J.; Baek, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, J.T.; Zhang, J.Y. NiCo2S4@Graphene as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.G.; Wang, H.L.; Liang, Y.Y.; Wu, J.Z.; Zhou, J.G.; Wang, J.; Regier, T.; Wei, F.; Dai, H.J. An Advanced Ni−Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.Q.; Masa, J.; Xia, W.; Maljusch, A.; Willinger, M.G.; Calavel, G.; Xie, K.P.; Schlogl, R.; Schuhmann, W.; Muhlert, M. Spinel Mn−Co Oxide in N-Doped Carbon Nanotubes as a Bifunctional Electrocatalyst Synthesized by Oxidative Cutting. J. Am. Chem. Soc. 2014, 136, 7551–7554. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.F.; Fan, G.L.; Ma, Q.; Yang, L.; Li, F. Facile Synthesis and Enhanced Catalytic Performance of Graphene-Supported Ni Nanocatalyst from a Layered Double Hydroxide-Based Composite Precursor. J. Mater. Chem. A 2014, 2, 7880–7889. [Google Scholar] [CrossRef]
- Sumboja, A.; An, T.; Goh, H.Y.; Lubke, M.; Howard, D.P.; Xu, Y.; Handoko, A.D.; Zong, Y.; Liu, Z. One-Step Facile Synthesis of Cobalt Phosphides for Hydrogen Evolution Reaction Catalysts in Acidic and Alkaline Medium. ACS Appl. Mater. Interfaces 2018, 10, 15673–15680. [Google Scholar] [CrossRef] [PubMed]
- Pramana, S.S.; Cavallaro, A.; Li, C.; Handoko, A.D.; Chan, K.W.; Walker, R.J.; Regoutz, A.; Herrin, J.S.; Yeo, B.S.; Payne, D.J.; et al. Crystal Structure and Surface Characteristics of Sr-Doped GdBaCo2O6−δ Double Perovskites: Oxygen Evolution Reaction and Conductivity. J. Mater. Chem. A 2018, 6, 5335–5345. [Google Scholar] [CrossRef]
- Lübke, M.; Sumboja, A.; McCafferty, L.; Armer, C.F.; Handoko, A.D.; Du, Y.H.; McColl, K.; Cora, F.; Brett, D.; Liu, Z.L.; et al. Transition-Metal-Doped α-MnO2 Nanorods as Bifunctional Catalysts for Efficient Oxygen Reduction and Evolution Reactions. ChemistrySelect 2018, 3, 2613–2622. [Google Scholar] [CrossRef]
- Jia, G.; Hu, Y.; Qian, Q.; Yao, Y.; Zhang, S.; Li, Z.; Zou, Z. Formation of Hierarchical Structure Composed of (Co/Ni)Mn-LDH Nanosheets on MWCNT Backbones for Efficient Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 14527–14534. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.Y.; Nie, H.G.; Liu, Z.; Zhou, X.M.; Chen, X.A.; Huang, S.M. Sulfur-Doped Graphene as an Efficient Metal-Free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Gago, A.S.; Gochi-Ponce, Y.; Feng, J.Y.; Esquivel, J.P.; Sabate, N.; Santander, J.; Alonse-Vante, N. Tolerant Chalcogenide Cathodes of Membraneless Micro Fuel Cells. ChemSusChem 2012, 5, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Liang, Y.Y.; Li, Y.G.; Dai, H.J. Co1-xS-Graphene Hybrid: A High-Performance Metal Chalcogenide Electrocatalyst for Oxygen Reduction. Angew. Chem. Int. Ed. Engl. 2011, 50, 10969–10972. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Tao, L.; Hou, J.; Wang, S.Y.; Dai, L.M. Etched and Doped Co9S8/Graphene Hybrid for Oxygen Electrocatalysis. Energy Environ. Sci. 2016, 9, 1320–1326. [Google Scholar] [CrossRef]
- Chao, Y.S.; Tsai, D.S.; Wu, A.P.; Tseng, L.W.; Huang, Y.S. Cobalt Selenide Electrocatalyst Supported by Nitrogen-Doped Carbon and its Stable Activity toward Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2013, 38, 5655–5664. [Google Scholar] [CrossRef]
- Kong, D.S.; Wang, H.T.; Lu, Z.Y.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Rreaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.H.; Sumboja, A.; Zang, W.J.; Xie, J.P.; Gao, D.Q.; Pennycook, S.J.; Liu, Z.L.; Guan, C.; Wang, J. Integrated Hierarchical Carbon Flake Arrays with Hollow P-Doped CoSe2 Nanoclusters as an Advanced Bifunctional Catalyst for Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1804846. [Google Scholar] [CrossRef]
- Chen, P.R.; Yang, F.K.; Kostka, A.; Xia, W. Interaction of Cobalt Nanoparticles with Oxygen- and Nitrogen-Functionalized Carbon Nanotubes and Impact on Nitrobenzene Hydrogenation Catalysis. ACS Catal. 2014, 4, 1478–1486. [Google Scholar] [CrossRef]
- Mao, S.; Wen, Z.H.; Huang, T.Z.; Hou, Y.; Chen, J.H. High-Performance Bi-Functional Electrocatalysts of 3D Crumpled Graphene–Cobalt Oxide Nanohybrids for Oxygen Reduction and Evolution Reactions. Energy Environ. Sci. 2014, 7, 609–616. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Li, Y.G.; Wang, H.L.; Zhou, J.G.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 Nanocrystals on Graphene as a Synergistic Catalyst for Oxygen Reduction Reaction. Nat. Mater. 2011, 101, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Wang, C.M.; Wu, Z.Y.; Xiong, Y.J.; Xu, Q.; Yu, Y.H.; Jiang, H.L. From Bimetallic Metal-Organic Framework to Porous Carbon: High Surface Area and Multicomponent Active Dopants for Excellent Electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal−Organic Framework Derived Hybrid Co3O4-Carbon Porous Nanowire Arrays as Reversible Oxygen Evolution Electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.H.; Lou, Y.; He, S.; Tang, P.G.; Li, D.Q.; Alonso-Vante, N.; Feng, Y.J. Electrocatalytic Cobalt Nanoparticles Interacting with Nitrogen-Doped Carbon Nanotube in Situ Generated from a Metal-Organic Framework for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Sumboja, A.; Ma, Y.Y.; Zhang, H.; Wu, Y.; Wu, S.S.; Wu, H.J.; Liu, Z.L.; Guan, C.; Wang, J.; et al. Single Co Atoms Anchored in Porous N-Doped Carbon for Efficient Zinc−Air Battery Cathodes. ACS Catal. 2018, 8, 8961–8969. [Google Scholar] [CrossRef]
- Guan, C.; Sumboja, A.; Zang, W.J.; Qian, Y.H.; Zhang, H.; Liu, X.M.; Liu, Z.L.; Zhao, D.; Pennycook, S.J.; Wang, J. Decorating Co/CoNx Nanoparticles in Nitrogen-Doped Carbon Nanoarrays for Flexible and Rechargeable Zinc-Air Batteries. Energy Storage Mater. 2018, 16, 243–250. [Google Scholar] [CrossRef]
- Fan, G.L.; Li, F.; Evans, D.G.; Duan, X. Catalytic Applications of Layered Double Hydroxides: Recent Advances and Perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Zhang, Y.; Jiang, W.J.; Liu, X.; Xu, S.L.; Hou, R.J.; Zhang, F.Z.; Hu, J.S. Co@N-CNTs Derived from Triple-Role CoAl-Layered Double Hydroxide as an Efficient Catalyst for Oxygen Reduction Reaction. Carbon 2016, 107, 162–170. [Google Scholar] [CrossRef]
- Zhong, H.H.; Tian, R.; Gong, X.M.; Li, D.Q.; Tang, P.G.; Alonso-Vante, N.; Feng, Y.J. Advanced Bifunctional Electrocatalyst Generated through Cobalt Phthalocyanine Tetrasulfonate Intercalated Ni2Fe-Layered Double Hydroxides for a Laminar Flow Unitized Regenerative Micro-Cell. J. Power Sources 2017, 361, 21–30. [Google Scholar] [CrossRef]
- Wang, J.H.; Cui, W.; Liu, Q.; Xing, Z.C.; Asiri, A.M.; Sun, X.P. Recent Progress in Cobalt-Based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.F.; Qiao, J.L.; Zhou, X.J.; Shi, J.J.; Xu, P.; Zhang, L.; Zhang, J.J. Effects of Heat-Treatment and Pyridine Addition on the Catalytic Activity of Carbon-Supported Cobalt-Phthalocyanine for Oxygen Reduction Reaction in Alkaline Electrolyte. Int. J. Electrochem. Sci. 2013, 8, 3160–3175. [Google Scholar]
- Zhao, S.; Rasimick, B.; Mustain, W.; Xu, H. Highly Durable and Active Co3O4 Nanocrystals Supported on Carbon Nanotubes as Bifunctional Electrocatalysts in Alkaline Media. Appl. Catal. B 2017, 203, 138–145. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, L.J.; Ahnb, H.S.; Manthiram, A. Delineating the Roles of Co3O4 and N-Doped Carbon Nanoweb (CNW) in Bifunctional Co3O4/ CNW Catalysts for Oxygen Reduction and Oxygen Evolution Reactions. J. Mater. Chem. A 2015, 3, 11615–11623. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.C.; Wen, L.; Gao, L.B.; Zhao, J.P.; Chen, Z.P.; Zhou, G.M.; Li, F.; Cheng, H.M. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.P.; Kumaraguru, S.P.; Colon-Mercado, H.; Kim, H.; Popov, B.N.; Black, T.; Chen, D.A. Studies on Co-Based Catalysts Supported on Modified Carbon Substrates for PEMFC Cathodes. J. Power Sources 2006, 157, 56–63. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-Warthan, A.A.; Tremel, W. Graphene Based Metal and Metal Oxide Nanocomposites: Synthesis, Properties and their Applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Alonso-Vante, N. Platinum and Non-Platinum Nanomaterials for the Molecular Oxygen Reduction Reaction. Chemphyschem 2010, 11, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Tributsch, H.; Alonso-Vante, N. Energy Conversion Catalysis Using Semiconducting Transition Metal Cluster Compounds. Nature 1986, 323, 431–432. [Google Scholar]
- Higgins, D.C.; Hassan, F.M.; Seo, M.H.; Choi, J.Y.; Hoque, M.A.; Lee, D.U.; Chen, Z. Shape-Controlled Octahedral Cobalt Disulfide Nanoparticles Supported on Nitrogen and Sulfur-Doped Graphene/Carbon Nanotube Composites for Oxygen Reduction in Acidic Electrolyte. J. Mater. Chem. A 2015, 3, 6340–6350. [Google Scholar] [CrossRef]
- Delacôte, C.; Lewera, A.; Pisarek, M.; Kulesza, P.J.; Zelenay, P.; Alonso-Vante, N. The Effect of Diluting Ruthenium by Iron in RuxSey Catalyst for Oxygen Reduction. Electrochim. Acta 2010, 55, 7575–7580. [Google Scholar] [CrossRef]
- Lee, K.; Zhang, L.; Zhang, J.J. Ternary Non-Noble Metal Chalcogenide (W–Co–Se) as Electrocatalyst for Oxygen Reduction Reaction. Electrochem. Commun. 2007, 9, 1704–1708. [Google Scholar] [CrossRef]
- Zhao, C.; Li, D.Q.; Feng, Y.J. Size-Controlled Hydrothermal Synthesis and High Electrocatalytic Performance of CoS2 Nanocatalysts as Non-Precious Metal Cathode Materials for Fuel Cells. J. Mater. Chem. A 2013, 1, 5741–5746. [Google Scholar] [CrossRef]
- Alonso-Vante, N.; Malakhov, I.V.; Nikitenko, S.G.; Savinova, E.R.; Kochubey, D.I. The Structure Analysis of the Active Centers of Ru-containing Electrocatalysts for the Oxygen Reduction. An in Situ EXAFS Study. Electrochim. Acta 2002, 47, 3807–3814. [Google Scholar] [CrossRef]
- Behret, H.; Binder, H.; Sandstede, G. Electrocatalytic Oxygen Reduction with Thiospinels and Other Sulphides of Transition Metals. Electrochim. Acta 1975, 20, 111–117. [Google Scholar] [CrossRef]
- Vayner, E.; Sidik, R.A.; Anderson, A.B.; Popov, B.N. Experimental and Theoretical Study of Cobalt Selenide as a Catalyst for O2 Electroreduction. J. Phys. Chem. C 2017, 111, 10508–10513. [Google Scholar] [CrossRef]
- Baresel, D.; Sarholz, W.; Scharner, P.; Schmitz, J. Transition MetalChalcogenides as Oxygen Catalysts for Fuel Cells. Ber. Bunsenges. Phys. Chem. 1974, 78, 608–618. [Google Scholar]
- Sidik, R.A.; Anderson, A.B. Co9S8 as a Catalyst for Electroreduction of O2: Quantum Chemistry Predictions. J. Phys. Chem. B 2006, 110, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Y.; Aoki, Y.; Habazaki, H. Co9S8 Nanoparticles Incorporated in Hierarchically Porous 3D Few-Layer Graphene-Like Carbon with S, N-Doping as Superior Electrocatalyst for Oxygen Reduction Reaction. Part. Part. Syst. Charact. 2017, 34, 1700296. [Google Scholar] [CrossRef]
- Feng, Y.J.; Alonso-Vante, N. Structure Phase Transition and Oxygen Reduction Activity in Acidic Medium of Carbon-Supported Cobalt Selenide Nanoparticles. ECS Trans. 2009, 25, 167–173. [Google Scholar]
- Feng, Y.J.; Alonso-Vante, N. Carbon-Supported CoSe2 Nanoparticles for Oxygen Reduction Reaction in Acid Medium. Fuel Cells 2010, 10, 77–83. [Google Scholar]
- Wu, G.; Chung, H.T.; Nelson, M.; Artyushkova, K.; More, K.L.; Johnston, C.M.; Zelenay, P. Graphene-Enriched Co9S8-N-C Non-Precious Metal Catalyst for Oxygen Reduction in Alkaline Media. ECS Trans. 2011, 41, 1709–1717. [Google Scholar]
- Apostolova, R.D.; Shembel, E.M.; Talyosef, I.; Grinblat, J.; Markovsky, B.; Aurbach, D. Study of Electrolytic Cobalt Sulfide Co9S8 as an Electrode Material in Lithium Accumulator Prototypes. Russ. J. Electrochem. 2009, 45, 311–319. [Google Scholar] [CrossRef]
- Handoko, A.D.; Deng, S.; Deng, Y.; Cheng, A.W.F.; Chan, K.W.; Tan, H.R.; Pan, Y.; Tok, E.S.; Sow, C.H.; Yeo, B.S. Enhanced Activity of H2O2-Treated Copper(II) Oxide Nanostructures for the Electrochemical Evolution of Oxygen. Catal. Sci. Technol. 2016, 6, 269–274. [Google Scholar] [CrossRef]
- Antolini, E. Structural Parameters of Supported Fuel Cell Catalysts: The Effect of Particle Size, Inter-Particle Distance and Metal Loading on Catalytic Activity and Fuel Cell Performance. Appl. Catal. B 2016, 181, 298–313. [Google Scholar] [CrossRef]
- Wang, J.X.; Inada, H.; Wu, L.J.; Zhu, Y.M.; Choi, Y.M.; Liu, P.; Zhou, W.P.; Adzic, R.R. Oxygen Reduction on Well-Defined Core-Shell Nanocatalysts: Particle Size, Facet, and Pt Shell Thickness Effects. J. Am. Chem. Soc. 2009, 131, 17298–17302. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.J.; He, T.; Alonso-Vante, N. In Situ Free-surfactant Synthesis and ORR-electrochemistry of Carbon-Supported Co3S4 and CoSe2 Nanoparticles. Chem. Mater. 2008, 20, 26–28. [Google Scholar] [CrossRef]
- Feng, Y.J.; Alonso-Vante, N. Carbon-Supported Cubic CoSe2 Catalysts for Oxygen Reduction Reaction in Alkaline Medium. Electrochim. Acta 2012, 72, 129–133. [Google Scholar] [CrossRef]
- Feng., Y.J.; He, T.; Alonso-Vante, N. Oxygen Reduction Reaction on Carbon-Supported CoSe2 Nanoparticles in an Acidic Medium. Electrochim. Acta 2009, 54, 5252–5256. [Google Scholar] [CrossRef]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent Progress on Nitrogen/Carbon Structures Designed for Use in Energy and Sustainability Applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Zhou, W.J.; Lu, L.; Zhou, K.; Yang, L.J.; Ke, Y.T.; Tang, Z.H.; Chen, S.W. CoSe2 Nanoparticles Embedded Defective Carbon Nanotubes Derived from MOFs as Efficient Electrocatalyst for Hydrogen Evolution Reaction. Nano Energy 2016, 28, 143–150. [Google Scholar] [CrossRef]
- Alonso-Vante, N. Photocatalysis an Enhancer of Electrocatalytic Process, Current Opinion in Electrochemistry. Curr. Opin. Electrochem. 2018, 9, 114–120. [Google Scholar] [CrossRef]
- Unni, S.M.; Mora-Hernandez, J.M.; Kurungot, S.; Alonso-Vante, N. CoSe2 Supported on Nitrogen-Doped Carbon Nanohorns as a Methanol-Tolerant Cathode for Air-Breathing Microlaminar Flow Fuel Cells. ChemElectroChem 2015, 2, 1339–1345. [Google Scholar] [CrossRef]
- García-Rosado, I.J.; Uribe-Calderón, J.; Alonso-Vante, N. Nitrogen-Doped Reduced Graphite Oxide as a Support for CoSe Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media. J. Electrochem. Soc. 2017, 164, F658–F666. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.Q.; Tan, P.F.; Qiao, L.L.; Liu, Y.; Ma, Y.J.; Pan, J. Sulphur and Nitrogen Dual-Doped Mesoporous Carbon Hybrid Coupling with Graphite Coated Cobalt and Cobalt Sulfide Nanoparticles: Rational Synthesis and Advanced Multifunctional Electrochemical Properties. J. Colloid Interface Sci. 2018, 509, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Han, L.; Yu, M.; Wang, Z.Y.; Lou, X.W. Metal–Organic-Framework-Engaged Formation of Co Nanoparticle-Embedded Carbon@Co9S8 Double-Shelled Nanocages for Efficient Oxygen Reduction. Energy Environ. Sci. 2016, 9, 107–111. [Google Scholar] [CrossRef]
- Chen, B.L.; Li, R.; Ma, G.P.; Gou, X.L.; Zhu, Y.Q.; Xia, Y.D. Cobalt Sulfide/N, S Codoped Porous Carbon Core-Shell Nanocomposites as Superior Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reactions. Nanoscale 2015, 7, 20674–20684. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.Q.; Zai, J.T.; Wang, K.X.; Zhang, H.J.; Xu, M.; Shen, J.; Su, Y.Z.; Qian, X.F. Co3O4 Nanorods/Graphene Nanosheets Nanocomposites for Lithium Ion Batteries with Improved Reversible Capacity and Cycle Stability. J. Power Sources 2012, 202, 230–235. [Google Scholar] [CrossRef]
- Bai, G.M.; Dai, H.X.; Deng, J.G.; Liu, Y.X.; Wang, F.; Zhao, Z.X.; Qiu., W.G.; Au, C.T. Porous Co3O4 Nanowires and Nanorods: Highly Active Catalysts for the Combustion of Toluene. Appl. Catal. A 2013, 450, 42–49. [Google Scholar] [CrossRef]
- Liu, J.P.; Jiang, J.; Cheng, C.W.; Li, H.X.; Zhang, J.X.; Gong, H.; Fan, H.J. Co3O4 Nanowire@MnO2 Ultrathin Nanosheet Core/Shell Arrays: A New Class of High-Performance Pseudocapacitive Materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.Y.; Xiao, D. Synthesis of 3D-Nanonet Hollow Structured Co3O4 for High Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Dhavale, V.M.; Kurungot, S. Surface-Tuned Co3O4 Nanoparticles Dispersed on Nitrogen-Doped Graphene as an Efficient Cathode Electrocatalyst for Mechanical Rechargeable Zinc-Air Battery Application. ACS Appl. Mater. Interfaces 2015, 7, 21138–21149. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.D.; Gao, S.J.; Liu, T.Y.; Li, D.Q.; Tang, P.G.; Feng, Y.J. Controllable Synthesis and Bi-Functional Electrocatalytic Performance towards Oxygen Electrode Reactions of Co3O4/N-RGO Composites. Electrochim. Acta 2017, 226, 104–112. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.H.; Zhang, B.S.; Jin, J.T.; Su, D.S.; Wang, S.L.; Sun, G.Q. Controllable Synthesis of Cobalt Monoxide Nanoparticles and the Size-Dependent Activity for Oxygen Reduction Reaction. ACS Catal. 2014, 4, 2998–3001. [Google Scholar] [CrossRef]
- Wang, J.K.; Gao, R.; Zhou, D.; Chen, Z.j.; Wu, Z.H.; Schumacher, G.; Hu, Z.B.; Liu, X.F. Boosting the Electrocatalytic Activity of Co3O4 Nanosheets for a Li-O2 Battery through Modulating Inner Oxygen Vacancy and Exterior Co3+/Co2+ Ratio. ACS Catal. 2017, 7, 6533–6541. [Google Scholar] [CrossRef]
- Xu, J.B.; Gao, P.; Zhao, T.S. Non-Precious Co3O4 Nano-Rod Electrocatalyst for Oxygen Reduction Reaction in Anion-Exchange Membrane Fuel Cells. Energy Environ. Sci. 2012, 5, 5333–5339. [Google Scholar] [CrossRef]
- Odedairo, T.; Yan, X.C.; Ma, J.; Jiao, Y.L.; Yao, X.D.; Du, A.J.; Zhu, Z.H. Nanosheets Co3O4 Interleaved with Graphene for Highly Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2015, 7, 21373–21380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Li, Y.N.; Wang, M.Q.; Xu, M.W.; Bao, S.J. Hollow Co3O4 Nanocages Decorated Graphene Aerogels Derived from Carbon Wrapped Nano-Co for Efficient Oxygen Reduction Reaction. ChemistrySelect 2017, 2, 6359–6363. [Google Scholar] [CrossRef]
- Wu, Z.X.; Wang, J.; Han, L.L.; Lin, R.Q.; Liu, H.F.; Xin, H.L.L.; Wang, D.L. Supramolecular Gel-Assisted Synthesis of Double Shelled Co@CoO@N-C/C Nanoparticles with Synergistic Electrocatalytic Activity for the Oxygen Reduction Reaction. Nanoscale 2016, 8, 4681–4687. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Chen, X.; Evans, D.G.; Yang, W.S. Well-Dispersed Co3O4/Co2MnO4 Nanocomposites as a Synergistic Bifunctional Catalyst for Oxygen Reduction and Oxygen Evolution Reactions. Nanoscale 2013, 5, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhong, H.H.; Li, D.Q.; Tang, P.G.; Feng, Y.J. Reduced Graphene Oxide Supported CoO/MnO2 Electrocatalysts from Layered Double Hydroxides for Oxygen Reduction Reaction. Electrochim. Acta 2015, 173, 575–580. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Z.C.; Wu, X.Q.; Liu, X.W.; Li, M.G. Synergistic Effect between Strongly Coupled CoAl Layered Double Hydroxides and Graphene for the Electrocatalytic Reduction of Oxygen. Electrochim. Acta 2016, 192, 196–204. [Google Scholar] [CrossRef]
- Huo, R.J.; Jiang, W.J.; Xu, S.L.; Zhang, F.Z.; Hu, J.S. Co/CoO/CoFe2O4/G Nanocomposites Derived from Layered Double Hydroxides towards Mass Production of Efficient Pt-Free Electrocatalysts for Oxygen Reduction Reaction. Nanoscale 2014, 6, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, R. A New Fuel Cell Cathode Catalyst. Nature 1964, 21, 1212–1213. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, R.; Nallathambi, V.; Artyushkova, K.; Barton, S.C. Non-Precious Oxygen Reduction Catalysts Prepared by High-Pressure Pyrolysis for Low-Temperature Fuel Cells. Appl. Catal. B 2009, 92, 209–216. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A Class of Non-Precious Metal Composite Catalysts for Fuel Cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Morozan, A.; Jegou, P.; Jousselme, B.; Palacin, S. Electrochemical Performance of Annealed Cobalt-Benzotriazole/CNTs Catalysts towards the Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2011, 13, 21600–21607. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, F.; Zhang, N.L.; An, L.; Xia, D.G. Mechanism of Oxygen Reduction Reaction Catalyzed by Fe(Co)-Nx/C. Phys. Chem. Chem. Phys. 2013, 15, 19330–19336. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.Y.; Ramaswamy, N.; Tylus, U.; Strickland, K.; Li, J.K.; Serov, A.; Artyushkova, K.; Atanassov, P.; Anibal, J.; Gumeci, C.; et al. Spectroscopic Insights into the Nature of Active Sites in Iron–Nitrogen–Carbon Electrocatalysts for Oxygen Reduction in Acid. Nano Energy 2016, 29, 65–82. [Google Scholar] [CrossRef]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Recent Progress in Nitrogen-Doped Carbon and its Composites as Electrocatalysts for Fuel Cell Applications. Int. J. Hydrogen Energy 2013, 38, 9370–9386. [Google Scholar] [CrossRef]
- Niwa, H.; Horiba, K.; Harada, Y.; Oshima, M.; Ikeda, T.; Terakura, K.; Ozaki, J.; Miyata, S. X-ray Absorption Analysis of Nitrogen Contribution to Oxygen Reduction Reaction in Carbon Alloy Cathode Catalysts for Polymer Electrolyte Fuel Cells. J. Power Sources 2009, 187, 93–97. [Google Scholar] [CrossRef]
- Hu, C.G.; Xiao, Y.; Zhao, Y.; Chen, N.; Zhang, Z.P.; Cao, M.H.; Qu, L.T. Highly Nitrogen-Doped Carbon Capsules: Scalable Preparation and High-Performance Applications in Fuel Cells and Lithium Ion Batteries. Nanoscale 2013, 5, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Atanassov, P.; Kiefer, B. Catalytic Activity of Co–Nx/C Electrocatalysts for Oxygen Reduction Reaction: A Density Functional Theory Study. Phys. Chem. Chem. Phys. 2013, 15, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.Q.; Yao, T.; Wu, Y.; Zheng, L.R.; Lin, Y.; Liu, W.; Ju, H.X.; Zhu, J.F.; Hong, X.; Deng, Z.X.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. Engl. 2016, 55, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Amiinu, I.S.; Liu, X.B.; Pu, Z.H.; Li, W.Q.; Li, Q.D.; Zhang, J.; Tang, H.L.; Zhang, H.N.; Mu, S.C. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1704638–1704646. [Google Scholar] [CrossRef]
- Peng, H.L.; Liu, F.F.; Liu, X.J.; Liao, S.J.; You, C.H.; Tian, X.L.; Nan, H.X.; Luo, F.; Song, H.Y.; Fu, Z.Y.; et al. Effect of Transition Metals on the Structure and Performance of the Doped Carbon Catalysts Derived From Polyaniline and Melamine for ORR Application. ACS Catal. 2014, 4, 3797–3805. [Google Scholar] [CrossRef]
- Wiesener, K. N4-Chelates as Electrocatalyst for Cathodic Oxygen Reduction. Electrochim. Acta 1986, 31, 1073–1078. [Google Scholar] [CrossRef]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the Role of Metals in Nitrogen-Doped Carbon Electrocatalysts for Oxygen Reduction. Angew. Chem. Int. Ed. Engl. 2015, 54, 10102–10120. [Google Scholar] [CrossRef] [PubMed]
- Bagotzky, V.S.; Tarasevich, M.R.; Radyushkina, K.A.; Levina, O.A.; Andrusyova, S.I. Electrocatalysis of the Oxygen Reduction Process on Metal Chelates in Acid Electrolyte. J. Power Sources 1978, 2, 233–240. [Google Scholar] [CrossRef]
- Wiesener, K.; Ohms, D.; Neumann, V.; Franke, R. N4 Macrocycles as Electrocatalysts for the Cathodic Reduction of Oxygen. Mater. Chem. Phys. 1989, 22, 457–475. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yue, X.P.; Li, K.X.; Qiao, J.L.; Wilkinson, D.P.; Zhang, J.J. PEM Fuel Cell Electrocatalysts Based on Transition Metal Macrocyclic Compounds. Coord. Chem. Rev. 2016, 315, 153–177. [Google Scholar] [CrossRef]
- Osmieri, L.; Videla, A.H.A.M.; Specchia, S. Activity of Co–N Multi Walled Carbon Nanotubes Electrocatalysts for Oxygen Reduction Reaction in Acid Conditions. J. Power Sources 2015, 278, 296–307. [Google Scholar] [CrossRef]
- Nallathambi, V.; Lee, J.W.; Kumaraguru, S.P.; Wu, G.; Popov, B.N. Development of High Performance Carbon Composite Catalyst for Oxygen Reduction Reaction in PEM Proton Exchange Membrane Fuel Cells. J. Power Sources 2008, 183, 34–42. [Google Scholar] [CrossRef]
- Lalande, G.; Côté, R.; Tamizhmani, G.; Guay, D.; Dodelet, J.P.; Dignard-Bailey, L.; Weng, L.T.; Bertrand, P. Physical, Chemical and Electrochemical Characterization of Heat-Treated Tetracarboxylic Cobalt Phthalocyanine Adsorbed on Carbon Black as Electrocatalyst for Oxygen Reduction in Polymer Electrolyte Fuel Cells. Electrochim. Acta 1995, 40, 2635–2646. [Google Scholar] [CrossRef]
- Weng, L.T.; Bertrand, P.; Lalande, G.; Guay, D.; Dodelet, J.P. Surface Characterization by Time-of-Flight SIMS of a Catalyst for Oxygen Electroreduction: Pyrolyzed Cobalt Phthalocyanine-on-Carbon Black. Appl. Surf. Sci. 1985, 84, 9–21. [Google Scholar] [CrossRef]
- Jahnke, H.; Schōnborn, M.; Zirnmermann, G. Organic Dyestuffs as Catalysts for Fuel Cells. Top. Curr. Chem. 1976, 61, 133–181. [Google Scholar] [PubMed]
- Kong, A.G.; Kong, Y.Y.; Zhu, X.F.; Han, Z.; Shan, Y.K. Ordered Mesoporous Fe (or Co)–N–Graphitic Carbons as Excellent Non-Precious-Metal Electrocatalysts for Oxygen Reduction. Carbon 2014, 78, 49–59. [Google Scholar] [CrossRef]
- Tang, C.; Wang, B.; Wang, H.F.; Zhang, Q. Defect Engineering toward Atomic Co-Nx-C in Hierarchical Graphene for Rechargeable Flexible Solid Zn-Air Batteries. Adv. Mater. 2017, 29, 1703185–1703191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Wang, A.Q.; Wang, W.T.; Huang, Y.Q.; Liu, X.Y.; Miao, S.; Liu, J.Y.; Zhang, T. Co–N–C Catalyst for C–C Coupling Reactions: On the Catalytic Performance and Active Sites. ACS Catal. 2015, 5, 6563–6572. [Google Scholar] [CrossRef]
- Hu, F.; Yang, H.C.; Wang, C.H.; Zhang, Y.J.; Lu, H.; Wang, Q.B. Co-N-Doped Mesoporous Carbon Hollow Spheres as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction. Small 2017, 13, 1602507–1602514. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.C.; Meng, Z.H.; Tang, H.L.; Wang, Y.; Tsiakaras, P. 3D Co-N-doped Hollow Carbon Spheres as Excellent Bifunctional Electrocatalysts for Oxygen Reduction Reaction and Oxygen Evolution Reaction. Appl. Catal. B 2017, 217, 477–484. [Google Scholar] [CrossRef]
- Aijaz, A.; Masa, J.; Rosler, C.; Xia, W.; Weide, P.; Botz, A.J.R.; Fischer, R.A.; Schuhmann, W.; Muhler, M. Co@Co3O4 Encapsulated in Carbon Nanotube-Grafted Nitrogen-Doped Carbon Polyhedra as an Advanced Bifunctional Oxygen Electrode. Angew. Chem. Int. Ed. Engl. 2016, 55, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Wang, Q.; Han, P.; Zheng, G.F. Cu, Co-Embedded N-Enriched Mesoporous Carbon for Efficient Oxygen Reduction and Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700193–1700200. [Google Scholar] [CrossRef]
- Lin, Q.P.; Bu, X.H.; Kong, A.G.; Mao, C.Y.; Bu, F.; Feng, P.Y. Heterometal-Embedded Organic Conjugate Frameworks from Alternating Monomeric Iron and Cobalt Metalloporphyrins and Their Application in Design of Porous Carbon Catalysts. Adv. Mater. 2015, 27, 3431–3436. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.J.; Peng, Z.W.; Ye, R.Q.; Zhou, H.Q.; Guo, X. M3C (M: Fe, Co, Ni) Nanocrystals Encased in Graphene Nanoribbons: An Active and Stable Bifunctional Electrocatalyst for Oxygen Reduction and Hydrogen Evolution Reactions. ACS Nano 2015, 9, 7407–7418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Teo, M.; Wong, P.C.; Wong, K.C.; Narita, I.; Ernst, F.; Mitchell, K.A.R.; Campbell, S.A. Synthesis, Characterization of a CoSe2 Catalyst for the Oxygen Reduction Reaction. Appl. Catal. A 2010, 386, 157–165. [Google Scholar] [CrossRef]
- Qiao, X.C.; Jin, J.T.; Fan, H.B.; Li, Y.W.; Liao, S.J. In Situ Growth of Cobalt Sulfide Hollow Nanospheres Embedded in Nitrogen and Sulfur Co-doped Graphene Nanoholes as a Highly Active Electrocatalyst for Oxygen Reduction and Evolution. J. Mater. Chem. A 2017, 5, 12354–12360. [Google Scholar] [CrossRef]
- Liang, H.; Li, C.W.; Chen, T.; Cui, L.; Han, J.R.; Peng, Z.; Liu, J.Q. Facile Preparation of Three-Dimensional Co1-xS/Sulfur and Nitrogen-Codoped Graphene/Carbon Foam for Highly Efficient Oxygen Reduction Reaction. J. Power Sources 2018, 378, 699–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.W.; Yin, X.Y.; Yan, Y.; Zhan, K.; Yang, J.H.; Zhao, B. Cobalt Sulfide Supported on Nitrogen and Sulfur Dual-Doped Reduced Graphene Oxide for Highly Active Oxygen Reduction Reaction. RSC Adv. 2017, 7, 50246–50253. [Google Scholar] [CrossRef]
- Ganesan, P.; Prabu, M.; Sanetuntikul, J.; Shanmugam, S. Cobalt Sulfide Nanoparticles Grown on Nitrogen and Sulfur Codoped Graphene Oxide: An Efficient Electrocatalyst for Oxygen Reduction and Evolution Eeactions. ACS Catal. 2015, 5, 3625–3637. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.Z.; Jiang, J.; Liu, F.; Hou, Y.L. Multifunctional Co3S4/Graphene Composites for Lithium Ion Batteries and Oxygen Reduction Reaction. Chem. Eur. J. 2013, 19, 5183–5190. [Google Scholar] [CrossRef] [PubMed]
- Sennu, P.; Christy, M.; Aravindan, V.; Lee, Y.G.; Nahm, K.S.; Lee, Y.S. Two-Dimensional Mesoporous Cobalt Sulfide Nanosheets as a Superior Anode for a Li-Ion Battery and a Bifunctional Electrocatalyst for the LiO2 System. Chem. Mater. 2015, 27, 5726–5735. [Google Scholar] [CrossRef]
- Arunchander, A.; Peera, S.G.; Giridhar, V.V.; Sahu, A.K. Synthesis of Cobalt Sulfide-Graphene as an Efficient Oxygen Reduction Catalyst in Alkaline Medium and its Application in Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2016, 164, F71–F80. [Google Scholar] [CrossRef]
- Fu, S.F.; Zhu, C.Z.; Song, J.H.; Feng, S.; Du, D.; Engelhard, M.H.; Xiao, D.D.; Li, D.S.; Lin, Y.H. Two-Dimensional N, S-Codoped Carbon/Co9S8 Catalysts Derived from Co(OH)2 Nanosheets for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9, 36755–36761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Yao, H.B.; Wang, Y.; Liu, H.L.; Gao, M.R.; Shen, P.K.; Yu, S.H. Hierarchical Hollow Co9S8 Microspheres: Solvothermal Synthesis, Magnetic, Electrochemical, and Electrocatalytic Properties. Chemistry 2010, 16, 12000–12007. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Jing, F.; Xu, Z.X.; Zhang, F.; Mai, Y.Y.; Wu, D.Q. Highly Crumpled Hybrids of Nitrogen/Sulfur Dual-Doped Graphene and Co9S8 Nanoplates as Efficient Bifunctional Oxygen Electrocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 12340–12347. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, G.; Giannozzi, P.; Bonapasta, A.A.; Guidoni, L. Reaction Pathways for Oxygen Evolution Promoted by Cobalt Catalyst. J. Am. Chem. Soc. 2013, 135, 15353–15363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Mota, M.; Bajdich, M.; Viswanathan, V.; Vojvodic, A.; Bell, A.T.; Nørskov, J.K. Importance of Correlation in Determining Electrocatalytic Oxygen Evolution Activity on Cobalt Oxides. J. Phys. Chem. C 2012, 116, 21077–21082. [Google Scholar] [CrossRef]
- Bajdich, M.; Garcia-Mota, M.; Vojvodic, A.; Norskov, J.K.; Bell, A.T. Theoretical Investigation of the Activity of Cobalt Oxides for the Electrochemical Oxidation of Water. J. Am. Chem. Soc. 2013, 135, 13521–13530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.W.; Cheng, H.; Lyu, M.J.; Fan, S.J.; Liu, Q.H.; Zhang, W.S.; Zhi, Y.D.; Wang, C.M.; Xiao, C.; Wei, S.Q.; et al. Low Overpotential in Vacancy-Rich Ultrathin CoSe2 Nanosheets for Water Oxidation. J. Am. Chem. Soc. 2014, 136, 15670–15675. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.R.; Cao, X.; Gao, Q.; Xu, Y.F.; Zheng, Y.R.; Jiang, J.; Yu, S. Nitrogen-Doped Graphene Supported CoSe2 Nanobelt Composite Catalyst for Efficient Water Oxidation. ACS Nano 2014, 8, 3970–3978. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Yao, Z.Y.; Shang, C.S.; Wang, E.K. Amorphous Co2B Grown on CoSe2 Nanosheets as a Hybrid Catalyst for Efficient Overall Water Splitting in Alkaline Medium. ACS Appl. Mater. Interfaces 2017, 9, 39312–39317. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.E.G.; Brandon, M.P. The Oxygen Evolution Reaction on Passive Oxide Covered Transition Metal Electrodes in Alkaline Solution. Part III - Iron. Int. J. Electrochem. Sci. 2008, 3, 1425–1462. [Google Scholar]
- Yeo, B.S.; Bell, A.T. Enhanced Activity of Gold-Supported Cobalt Oxide for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.W.; Kellerman, E.; Leidheiser, H.J. In Situ Studies of the Passivation and Anodic Oxidation of Cobalt by Emission Moessbauer Spectroscopy. J. Electrochem. Soc. 1976, 123, 1276–1284. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.Y.; Han, Y.Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z.H. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via A Two-Electron Pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Barkaoui, S.; Haddaoui, M.; Dhaouadi, H.; Raouafi, N.; Touati, F. Hydrothermal Synthesis of Urchin-Like Co3O4 Nanostructures and their Electrochemical Sensing Performance of H2O2. J. Solid State Chem. 2015, 228, 226–231. [Google Scholar] [CrossRef]
- Du, S.C.; Ren, Z.Y.; Qu, Y.; Wu, J.; Xi, W.; Zhu, J.Q.; Fu, H.G. Co3O4 Nanosheets as a High-Performance Catalyst for Oxygen Evolution Proceeding via a Double Two-Electron Process. Chem. Commun. 2016, 52, 6705–6708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zhou, T.; Jiang, K.; Da, P.M.; Peng, Z.; Tang, J.; Kong, B.A.; Cai, W.B.; Yang, Z.Q.; Zheng, G.F. Reduced Mesoporous Co3O4 Nanowires as Efficient Water Oxidation Electrocatalysts and Supercapacitor Electrodes. Adv. Energy Mater. 2014, 4, 1400696–1400702. [Google Scholar] [CrossRef]
- Xia, Y.S.; Dai, H.X.; Jiang, H.Y.; Zhang, L. Three-Dimensional Ordered Mesoporous Cobalt Oxides: Highly Active Catalysts for the Oxidation of Toluene and Methanol. Catal. Commun. 2010, 11, 1171–1175. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Chen, S.Q.; Sun, B.; Su, D.W.; Huang, X.D.; Liu, H.; Yan, Y.M.; Sun, K.N.; Wang, G.X. Graphene-Co3O4 Nanocomposite as Electrocatalyst with High Performance for Oxygen Evolution Reaction. Sci. Rep. 2015, 5, 7629–7635. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.R.; Bandal, H.A.; Tamboli, A.H.; Kim, H. Environment Friendly Hydrothermal Synthesis of Carbon–Co3O4 Nanorods Composite as an Efficient Catalyst for Oxygen Evolution Reaction. J. Energy Chem. 2017, 26, 695–702. [Google Scholar] [CrossRef]
- Du, S.C.; Ren, Z.Y.; Zhang, J.; Wu, J.; Xi, W.; Zhu, J.Q.; Fu, H.G. Co3O4 Nanocrystal Ink Printed on Carbon Fiber Paper as a Large-Area Electrode for Electrochemical Water Splitting. Chem. Commun. 2015, 51, 8066–8069. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Ren, X.; Ma, H.M.; Sun, X.; Zhang, Y.; Kuang, X.; Yan, T.; Ju, H.X.; Wu, D.; Wei, Q. CoC2O4·2H2O Derived Co3O4 Nanorods Array: A High-Efficiency 1D Electrocatalyst for Alkaline Oxygen Evolution Reaction. Chem. Commun. 2018, 54, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.W.; Yin, H.J.; You, T.T.; Zheng, L.R.; Zhang, J.; Wang, P.X.; Jin, Z.; Tian, Y.; Liu, J.Z.; Tang, Z.Y.; et al. Efficient Electrocatalytic Water Oxidation by Using Amorphous Ni-Co Double Hydroxides Nanocages. Adv. Energy Mater. 2015, 5, 1401880–1401887. [Google Scholar] [CrossRef]
- Surendranath, Y.; Lutterman, D.A.; Liu, Y.; Nocera, D.G. Nucleation, Growth, and Repair of Acobalt-Based Oxygen Evolving Catalyst. J. Am. Chem. Soc. 2012, 134, 6326–6336. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Wang, X. Edge Overgrowth of Spiral Bimetallic Hydroxides Ultrathin-Nanosheets for Water Oxidation. Chem. Sci. 2015, 6, 3572–3576. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ren, Z.Y.; Du, S.C.; Kong, L.J.; Liu, B.W.; Xi, W.; Zhu, J.Q.; Fu, H.G. A Highly Active Oxygen Evolution Electrocatalyst: Ultrathin CoNi Double Hydroxide/CoO Nanosheets Synthesized via Interface-Directed Assembly. Nano Res. 2016, 9, 713–725. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Jarvis, K.A.; Therese, S.; Ferreira, P.J.; Manthiram, A. Spinel-Type Lithium Cobalt Oxide as a Bifunctional Electrocatalyst for the Oxygen Evolution and Oxygen Reduction Reactions. Nat. Commun. 2014, 5, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Lu, Z.Y.; Xu, T.H.; Wu, X.C.; Tian, Y.; Li, Y.P.; Huo, Z.Y.; Sun, X.M.; Duan, X. Trinary Layered Double Hydroxides as High-Performance Bifunctional Materials for Oxygen Electrocatalysis. Adv. Energy Mater. 2015, 5, 1500245–1500250. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.L.; Risch, M.; Hong, W.T.; Zhou, J.G.; Shao-Horn, Y. Double Perovskites as a Family of Highly Active Catalysts for Oxygen Evolution in Alkaline Solution. Nat. Commun. 2013, 4, 2439–2745. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.S.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yamaguchi, H.; Li, J.W.; Silva, R.; Alves, D.C.B.; Fujita, T.; Chen, M.W.; Asefa, T.; Shenoy, V.B.; Eda, G.; et al. Enhanced Catalytic Activity in Strained Chemically Exfoliated WS2 Nanosheets for Hydrogen Evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yan, Y.X.; Liu, Q.; Wang, J.; Yang, B.; Wang, B.; Jing, X.Y.; Liu, L.H. Exfoliation at Room Temperature for Improving Electrochemical Performance for Supercapacitors of Layered MnO2. J. Electrochem. Soc. 2013, 161, E1–E5. [Google Scholar] [CrossRef]
- Song, F.; Hu, X.L. Exfoliation of Layered Double Hydroxides for Enhanced Oxygen Evolution Catalysis. Nat. Commun. 2014, 5, 4477–4485. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.F.; Meng, F.; Caban-Acevedo, M.; Li, L.S.; Forticaux, A.; Xiu, L.C.; Wang, Z.C.; Jin, S. Hydrothermal Continuous Flow Synthesis and Exfoliation of NiCo Layered Double Hydroxide Nanosheets for Enhanced Oxygen Evolution Catalysis. Nano Lett. 2015, 15, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yan, L.T.; Luo, H.; Mustain, W.; Xu, H. Recent Progress and Perspectives of Bifunctional Oxygen Reduction/Evolution Catalyst Development for Regenerative Anion Exchange Membrane Fuel Cells. Nano Energy 2018, 47, 172–198. [Google Scholar] [CrossRef]
- Mamlouk, M.; Kumar, S.M.S.; Gouerec, P.; Scott, K. Electrochemical and Fuel Cell Evaluation of Co Based Catalyst for Oxygen Reduction in Anion Exchange Polymer Membrane Fuel Cells. J. Power Sources 2011, 196, 7594–7600. [Google Scholar] [CrossRef]
- Wu, X.; Scott, K. A Non-Precious Metal Bifunctional Oxygen Electrode for Alkaline Anion Exchange Membrane Cells. J. Power Sources 2012, 206, 14–19. [Google Scholar] [CrossRef]
- Kong, D.S.; Cha, J.J.; Wang, H.T.; Lee, H.R.; Cui, Y. First-Row Transition Metal Dichalcogenide Catalysts for Hydrogen Evolution Reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.L.; Hu, J.M.; Asiri, A.M.; Luo, Y.L.; Sun, X.P. CoSe2 Nanowires Array as a 3D Electrode for Highly Efficient Electrochemical Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 3877–3881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Yang, B.; Wu, X.L.; Li, Z.J.; Lei, L.C.; Zhang, X.W. Polymorphic CoSe2 with Mixed Orthorhombic and Cubic Phases for Highly Efficient Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Qian, X.; Zhu, C.L.; Jiang, X.X.; Shao, L.; Hou, L.X. Template Synthesis of CoSe2/Co3Se4 Nanotubes: Tuning of their Crystal Structures for Photovoltaics and Hydrogen Evolution in Alkaline Medium. J. Mater. Chem. A 2017, 5, 4513–4526. [Google Scholar] [CrossRef]
- Wang, K.; Xi, D.; Zhou, C.J.; Shi, Z.Q.; Xia, H.Y.; Liu, G.W.; Qiao, G.J. CoSe2 Necklace-Like Nanowires Supported by Carbon Fiber Paper: A 3D Integrated Electrode for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 9415–9420. [Google Scholar] [CrossRef]
- Yue, H.H.; Yu, B.; Qi, F.; Zhou, J.H.; Wang, X.Q.; Zheng, B.J.; Zhang, W.L.; Li, Y.R.; Chen, Y.F. Interwoven CoSe2/CNTs Hybrid as a Highly Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2017, 253, 200–207. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, G.D.; Kim, J.H.; Park, S.K.; Kang, Y.C. Rational Design and Synthesis of Extremely Efficient Macroporous CoSe2-CNT Composite Microspheres for Hydrogen Evolution Reaction. Small 2017, 13, 1700068. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, X.K.; Nie, Y.L.; Tian, C.; Yang, C.; Zhou, Z.X.; Li, Y.; Gao, X.Y. Successful Synthesis of 3D CoSe2 Hollow Microspheres with High Surface Roughness and its Excellent Performance in Catalytic Hydrogen Evolution Reaction. Chem. Eng. J. 2017, 321, 105–112. [Google Scholar] [CrossRef]
- Xiao, H.Q.; Wang, S.T.; Wang, C.; Li, Y.Y.; Zhang, H.R.; Wang, Z.J.; Zhou, Y.; An, C.H.; Zhang, J. Lamellar Structured CoSe2 Nanosheets Directly Arrayed on Ti Plate as an Efficient Electrochemical Catalyst for Hydrogen Evolution. Electrochim. Acta 2016, 217, 156–162. [Google Scholar] [CrossRef]
- Di, J.; Yan, C.; Handoko, A.D.; Seh, Z.W.; Li, H.M.; Liu, Z. Ultrathin Two-Dimensional Materials for Photo- and Electrocatalytic Hydrogen Evolution. Mater. Today 2018, 21, 749–770. [Google Scholar] [CrossRef]
- Liu, Y.W.; Hua, X.M.; Xiao, C.; Zhou, T.F.; Huang, P.C.; Guo, Z.P.; Pan, B.C.; Xie, Y. Heterogeneous Spin States in Ultrathin Nanosheet Inducing Subtle Lattice Distortion for Efficiently Triggering Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Li, F.; Li, W.Z.; Gao, W.B.; Wen, H.; Li, J.; Hu, Y.P.; Luo, Y.T.; Li, R. Porous CoS2 Nanostructures Based on ZIF-9 Supported on Reduced Graphene Oxide: Favourable Electrocatalysis for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2017, 42, 6665–6673. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.W.; Zang, Y.P.; Liu, R.R.; Liu, G.Q.; Wang, G.Z.; Zhang, Y.X.; Zhang, H.M.; Zhao, H.J. Co/Co9S8@S, N-Doped Porous Graphene Sheets Derived from S, N Dual Organic Ligands Assembled Co-MOFs as Superior Electrocatalysts for Full Water Splitting in Alkaline Media. Nano Energy 2016, 30, 93–102. [Google Scholar] [CrossRef]
- Lin, J.; He, J.R.; Qi, F.; Zheng, B.J.; Wang, X.Q.; Yu, B.; Zhou, K.R.; Zhang, W.L.; Li, Y.R.; Chen, Y.F. In-Situ Selenization of Co-based Metal-Organic Frameworks as a Highly Efficient Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2017, 247, 258–264. [Google Scholar] [CrossRef]
- Zhang, H.X.; Lei, L.C.; Zhang, X.W. One-Step Synthesis of Cubic Pyrite-Type CoSe2 at Low Temperature for Efficient Hydrogen Evolution Reaction. RSC Adv. 2014, 4, 54344–54348. [Google Scholar] [CrossRef]
- Li, Y.Z.; Niu, S.Q.; Rakov, D.; Wang, Y.; Caban-Acevedo, M.; Zheng, S.J.; Song, B.; Xu, P. Metal Organic Framework-Derived CoPS/N-doped Carbon for Efficient Electrocatalytic Hydrogen Evolution. Nanoscale 2018, 10, 7291–7297. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, X.; Li, G.D.; Wu, Y.Y.; Gao, R.Q.; Zou, X.X. Vertically Grown CoS Nanosheets on Carbon Cloth as Efficient Hydrogen Evolution Electrocatalysts. Int. J. Hydrogen Energy 2017, 42, 9914–9921. [Google Scholar] [CrossRef]
- Ouyang, C.B.; Wang, X.; Wang, S.Y. Phosphorus-Doped CoS2 Nanosheet Arrays as Ultra-Efficient Electrocatalysts for the Hydrogen Evolution Reaction. Chem. Commun. 2015, 51, 14160–14163. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Qian, X.; Xu, C.; Huang, S.W.; Zhu, C.L.; Jiang, X.C.; Shao, L.; Hou, L.X. Hierarchical Porous Co9S8/Nitrogen-Doped Carbon@MoS2 Polyhedrons as pH Universal Electrocatalysts for Highly Efficient Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 28394–28405. [Google Scholar] [CrossRef] [PubMed]






















| Catalysts | Mass Loading (mg cm−2) | Electrolyte | RPM (rpm) | jk (mA cm−2) | E1/2 (V/RHE) | Tafel Slope (mV dec−1) | Refs. |
|---|---|---|---|---|---|---|---|
| Cubic CoSe2/NCNH a | 0.214 | 0.1 M KOH | 1600 | 8.1 @ 0.8 V | 0.81 | 52 | [66] |
| Hexagonal CoSe/N–RGO b | 0.286 | 0.1 M KOH | 1600 | 2.9 @ 0.85 V | 0.86 | 56 | [67] |
| Co3C–GNRs c | - | 0.1 M KOH | 1600 | 4.6 @ 0.5 V | 0.77 | 41 | [119] |
| Cubic CoSe2/XC-72 Vulcan | 0.1 | 0.5 M H2SO4 | 2000 | 0.1 @ 0.8 V | - | 113 | [120] |
| Cubic CoSe2/XC-72 Vulcan | 0.22 | 0.1 M KOH | 1600 | - | 0.71 | - | [61] |
| Co1-xS/RGO | 0.285 | 0.1 M KOH | 1600 | 3.8 @ 0.7 V | 0.75 | - | [18] |
| 0.5 M H2SO4 | 1.1 @ 0.7 V | 0.59 | - | ||||
| Co1-xS/N–S–G d | 0.5 | 0.1 M KOH | 1600 | 15% higher than Pt/C @ 0.6 V | 0.86 | 58 | [121] |
| Co1-xS/SNG/CF e | 0.153 | 0.1 M KOH | 1600 | 4.3 @ 0.2 V | 0.83 | 85 | [122] |
| Co–S/NS–RGO f | 0.38 | 0.1 M KOH | 900 | - | 0.81 | - | [123] |
| CoS2/NS–GO | 0.25 | 0.1 M KOH | 1600 | 7.7 | 0.79 | 30 | [124] |
| CoS2/XC-72 | 0.1 | 0.1 M KOH | 1600 | 4.2 @ 0.4 V | 0.71 | 73 | [46] |
| Co3S4/G g | 0.051 | 0.1 M KOH | 1600 | 4.5 @ −1.1 V (vs. Ag/AgCl) | - | - | [125] |
| Co3S4 nanosheets | - | 0.1 M KOH | 1600 | - | −0.19 (vs. Hg/HgO) | - | [126] |
| Co3S4/C | 0.011 | 0.5 M H2SO4 | 1600 | - | 0.26 | - | [60] |
| Co9S8/G | 0.6 | 0.5 M H2SO4 | 1600 | 3.7 @ −0.1 V (vs. Ag/AgCl) | −0.11 (vs. Ag/AgCl) | 52 | [127] |
| Co9S8/N–S–C h | 0.1 | 0.1 M KOH | 1600 | - | 0.90 | 74 | [128] |
| Hollow Co9S8 microspheres | 0.61 | 0.5 M H2SO4 | 1600 | - | ~0.18 | - | [129] |
| Co9S8/N–S–GgC3N4 | 0.612 | 0.1 M KOH | 1600 | - | −0.10 (vs. Ag/AgCl) | - | [130] |
| Co3O4/N–rmGO i | 0.24 | 0.1 M KOH | 1600 | 5.0 @ 0.4 V | 0.83 | 42 | [25] |
| Co@N–CNTs–m j | 0.6 | 0.1 M KOH | 1600 | 6.0 | 0.85 | - | [32] |
| Co–S/G–3 | 0.08 | 0.1 M KOH | 1600 | 7.0 @ 0.85 V | 0.83 | 38 | [80] |
| Co3O4–SP/NGr–24h k | - | 0.1 M KOH | 1600 | - | 0.82 | 76 | [75] |
| Co@CoO@N–C/C | - | 0.1 M KOH | 1600 | - | 0.81 | 69 | [82] |
| Co3O4/N–RGO–3 | 0.1 | 0.1 M KOH | 1600 | 5.24 | 0.82 | 55 | [76] |
| L1G5 l CoAl–LDHs/RGO | 0.255 | 0.1 M KOH | 1600 | 5.1 @ 0.2 V | 0.71 | - | [85] |
| CoPc/C | 0.071 | 0.1 M KOH | 1500 | - | 0.03 (vs. SHE) | 62 | [35] |
| NGM–Co | 0.25 | 0.1 M KOH | 1600 | - | - | 58 | [112] |
| Co–N–mC | 0.285 | 0.1 M KOH | 1600 | 4.5 @ 0.8 V | 0.85 | 45 | [114] |
| Co–N@HCS m | 0.3 | 0.1 M KOH | 1600 | 4.8 @ 0.8 V | - | 56 | [115] |
| MOFs–800 | 0.335 | 0.1 M KOH | 1600 | 3.7 @ 0.7 V | 0.80 | 42 | [28] |
| Co@Co3O4/NC–1 | 0.21 | 0.1 M KOH | 1600 | - | 0.80 | 92 | [116] |
| CuCo@NC | 0.182 | 0.1 M KOH | 1600 | 4.4 @ 0.8 V | 0.88 | 80 | [117] |
| Catalysts | Mass Loading (mg cm−2) | Electrolyte | RPM (rpm) | Eonset mV vs. RHE | η@ 10 mA cm−2 (mV) | Tafel Slope (mV dec−1) | TOF (s−1) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Nanobelts CoSe2/N-doped RGO a | 0.2 | 0.1 M KOH | 1600 | - | 366 | 76 | - | [135] |
| Co1-xS/N–S–G b | 0.5 | 0.1 M KOH | 1600 | - | 371 | 63 | - | [121] |
| N–Co9S8/G c | 0.2 | 0.1 M KOH | 1600 | 1.51 | 409 | 83 | - | [19] |
| Co2B/CoSe2 | 0.4 | 1 M KOH | - | 320 | 56 | - | [136] | |
| Co3O4–NSs d | 1.76 | 1 M KOH | 1600 | 1.51 | 330 | 69 | - | [142] |
| Reduced Co3O4 NWs e | 0.136 | 1 M KOH | 1000 | 1.52 | 400 | 72 | - | [143] |
| G–Co3O4 | 0.189 | 1 M KOH | 1600 | 0.41 vs. Ag/AgCl | 313 | 56 | 0.45 @ 0.35 V | [145] |
| C–Co3O4–NRs f | 0.142 | 1 M KOH | - | 1.59 | 415 | 53 | - | [146] |
| Co3O4 NCs g | 0.35 | 1 M KOH | - | 1.52 | 350 @ 16.5 mA cm−2 | 101 | - | [147] |
| OA–Co3O4 NCs | 0.35 | 1 M KOH | - | 1.55 | - | 118 | - | [147] |
| Co3O4 NA/CF h | 1.9 | 1 M KOH | - | - | 308 @ 15 mA cm−2 | 71 | 0.65 @ 0.41 V | [148] |
| NiCo2.7(OH)x | 0.2 | 1 M KOH | 1600 | 1.48 | 350 | 65 | 0.18 @ 0.35 V | [149] |
| CoFe/C | - | 1 M KOH | 1600 | 1.45 | 300 | 61 | - | [151] |
| CoNi/C | - | 1 M KOH | 1600 | 1.56 | 360 | 39 | - | [151] |
| Co5Mn–LDH/MWCNT j | 0.283 | 1 M KOH | 1600 | - | 300 | 74 | 0.47 @ 0.35 V | [15] |
| CoNi LDH/CoO | 0.265 | 1 M KOH | 1600 | 1.48 | 300 | 123 | 1.40 @ 0.40 V | [152] |
| CoCo LDH/CoO | 0.265 | 1 M KOH | 1600 | 1.54 | 340 | 123 | 0.80 @ 0.40 V | [152] |
| O–NiCoFe–LDH | 0.12 | 0.1 M KOH | 1600 | - | 340 | 93 | 0.02 @ 0.30 V | [154] |
| NiFeOx/Co–Ny–C | 0.196 | 1 M KOH | 1600 | 1.47 | 310 | 60 | [33] | |
| NiCo–NS k | 0.07 | 1 M KOH | - | 1.52 @ 1 mA cm−2 | 334 | 41 | 0.01 @ 0.30 V | [159] |
| CoCo–NS k | 0.07 | 1 M KOH | - | 1.54 @ 1 mA cm−2 | 353 | 45 | - | [159] |
| Exfoliated NiCo LDH | 0.17 | 1 M KOH | - | - | 367 | 112 | - | [160] |
| Catalysts | Mass Loading (mg cm−2) | Electrolyte | RPM (rpm) | η@10 mA cm−2 (mV) | Tafel Slope (mV dec−1) | Refs. |
|---|---|---|---|---|---|---|
| CoSe2@DC a | 0.357 | 0.5 M H2SO4 | - | 132 | 82 | [64] |
| Co3C–GNRs b | 0.142 | 0.5 M H2SO4 | 1600 | 125 | 57 | [119] |
| Co2B/CoSe2 | 0.4 | 1 M KOH | - | 300 | 76 | [136] |
| Cubic CoSe2/GD c | 2.8 | 0.5 M H2SO4 | - | 200 | 42 | [179] |
| Cubic CoSe2 nanoparticles/CF d | 0.26 | 0.5 M H2SO4 | - | 137 | 42 | [21] |
| Interwoven CoSe2/CNT e | 0.54 | 0.5 M H2SO4 | - | 186 | 32 | [170] |
| CoSe2–CNT e | 0.255 | 0.5 M H2SO4 | 2000 | 174 | 38 | [171] |
| Orthorhombic CoSe2 nanotubes | 0.283 | 1 M KOH | - | 124 | 66 | [168] |
| Cubic CoSe2 nanotubes | 0.283 | 1 M KOH | - | 149 | 79 | [168] |
| Polymorphic CoSe/GD c | - | 0.5 M H2SO4 | - | 150 | 31 | [167] |
| CoSe2 hollow microspheres/rGO | 0.277 | 0.5 M H2SO4 | - | 250 | 55 | [172] |
| CoPS/NC f | 0.17 | 0.5 M H2SO4 | 2000 | 80 | 68 | [180] |
| CoPS/NC f | 0.17 | 1 M KOH | 2000 | 148 | 78 | [180] |
| Nanowires CoSe2/CF d | - | 0.5 M H2SO4 | - | 150 | 34 | [169] |
| Nanowires CoSe2/CC g | - | 0.5 M H2SO4 | - | 150 | 32 | [166] |
| Nanosheets CoSe2/Ti plate | 0.16 | 0.5 M H2SO4 | - | 165 | 39 | [173] |
| CoS/CC g | 3.77 | 1 M KOH | - | 197 | 98 | [181] |
| 0.5 M H2SO4 | - | 212 | 112 | |||
| CoS2/RGO h | 0.285 | 0.5 M H2SO4 | - | 18 | 75 | [176] |
| CoS2/P | - | 0.5 M H2SO4 | - | 67 | 50 | [182] |
| Co9S8/NC e @MoS2 | 0.283 | 1 M KOH | - | 67 | 60 | [183] |
| 0.5 M H2SO4 | - | 117 | 69 | |||
| 1 M PBS | - | 267 | 126 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Campos-Roldán, C.A.; Zhao, Y.; Zhang, S.; Feng, Y.; Alonso-Vante, N. Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction. Catalysts 2018, 8, 559. https://doi.org/10.3390/catal8110559
Zhong H, Campos-Roldán CA, Zhao Y, Zhang S, Feng Y, Alonso-Vante N. Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction. Catalysts. 2018; 8(11):559. https://doi.org/10.3390/catal8110559
Chicago/Turabian StyleZhong, Haihong, Carlos A. Campos-Roldán, Yuan Zhao, Shuwei Zhang, Yongjun Feng, and Nicolas Alonso-Vante. 2018. "Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction" Catalysts 8, no. 11: 559. https://doi.org/10.3390/catal8110559
APA StyleZhong, H., Campos-Roldán, C. A., Zhao, Y., Zhang, S., Feng, Y., & Alonso-Vante, N. (2018). Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction. Catalysts, 8(11), 559. https://doi.org/10.3390/catal8110559