Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting
Abstract
:1. Introduction
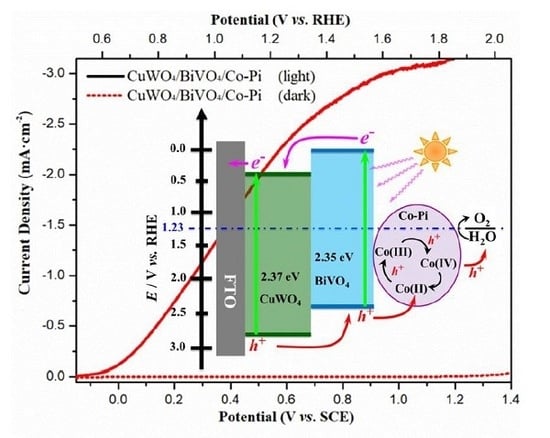
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Network-Structured CuWO4/BiVO4 and CuWO4/BiVO4/Co-Pi Nanocomposites
3.3. Characterization
3.4. Photoelectrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Steinmiller, E.M.P.; Choi, K.-S. Photochemical deposition of cobalt-based oxygen evolving catalyst on a semiconductor photoanode for solar oxygen production. Proc. Natl. Acad. Sci. USA 2009, 106, 20633–20636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Jin, T.; Diao, P.; Xu, D.; Wu, Q. High-aspect-ratio WO3 nanoneedles modified with nickel-borate for efficient photoelectrochemical water oxidation. Electrochim. Acta 2013, 114, 271–277. [Google Scholar] [CrossRef]
- Jin, T.; Diao, P.; Wu, Q.; Xu, D.; Hu, D.; Xie, Y.; Zhang, M. WO3 nanoneedles/α-Fe2O3/cobalt phosphate composite photoanode for efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2014, 148–149, 304–310. [Google Scholar] [CrossRef]
- Hu, D.; Diao, P.; Xu, D.; Wu, Q. Gold/WO3 nanocomposite photoanodes for plasmonic solar water splitting. Nano Res. 2016, 9, 1735–1751. [Google Scholar] [CrossRef]
- Jin, T.; Xu, D.; Diao, P.; He, W.-p.; Wang, H.-w.; Liao, S.-z. Tailored preparation of WO3 nano-grassblades on FTO substrate for photoelectrochemical water splitting. CrystEngComm 2016, 18, 6798–6808. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, D.; Xue, N.; Liu, T.; Xiang, M.; Diao, P. Photo-catalyzed surface hydrolysis of iridium(iii) ions on semiconductors: A facile method for the preparation of semiconductor/IrOx composite photoanodes toward oxygen evolution reaction. Phys. Chem. Chem. Phys. 2017, 19, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Diao, P.; Sun, J.; Xu, D.; Jin, T.; Xiang, M. Draining the photoinduced electrons away from an anode: The preparation of Ag/Ag3PO4 composite nanoplate photoanodes for highly efficient water splitting. J. Mater. Chem. A 2015, 3, 18991–18999. [Google Scholar] [CrossRef]
- Ding, C.; Shi, J.; Wang, D.; Wang, Z.; Wang, N.; Liu, G.; Xiong, F.; Li, C. Visible light driven overall water splitting using cocatalyst/BiVO4 photoanode with minimized bias. Phys. Chem. Chem. Phys. 2013, 15, 4589–4595. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.F.; van de Krol, R. Nature and Light Dependence of Bulk Recombination in Co-Pi-Catalyzed BiVO4 Photoanodes. J. Phys. Chem. C 2012, 116, 9398–9404. [Google Scholar] [CrossRef]
- Hu, D.; Diao, P.; Xu, D.; Xia, M.; Gu, Y.; Wu, Q.; Li, C.; Yang, S. Copper(ii) tungstate nanoflake array films: Sacrificial template synthesis, hydrogen treatment, and their application as photoanodes in solar water splitting. Nanoscale 2016, 8, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, F.; Zhao, F.; Han, N.; Li, Y. CuWO4 Nanoflake Array-Based Single-Junction and Heterojunction Photoanodes for Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 9211–9217. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Choi, K.-S. Synthesis and characterization of high surface area CuWO4 and Bi2WO6 electrodes for use as photoanodes for solar water oxidation. J. Mater. Chem. A 2013, 1, 5006–5014. [Google Scholar] [CrossRef]
- Pyper, K.J.; Yourey, J.E.; Bartlett, B.M. Reactivity of CuWO4 in Photoelectrochemical Water Oxidation Is Dictated by a Midgap Electronic State. J. Phys. Chem. C 2013, 117, 24726–24732. [Google Scholar] [CrossRef]
- Yourey, J.E.; Bartlett, B.M. Electrochemical deposition and photoelectrochemistry of CuWO4, a promising photoanode for water oxidation. J. Mater. Chem. 2011, 21, 7651–7660. [Google Scholar] [CrossRef]
- Yourey, J.E.; Pyper, K.J.; Kurtz, J.B.; Bartlett, B.M. Chemical Stability of CuWO4 for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2013, 117, 8708–8718. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Z.; Li, X.; Liu, Z. Promising Three-Dimensional Flowerlike CuWO4 Photoanode Modified with CdS and FeOOH for Efficient Photoelectrochemical Water Splitting. Ind. Eng. Chem. Res. 2018, 57, 6210–6217. [Google Scholar] [CrossRef]
- Nam, K.M.; Cheon, E.A.; Shin, W.J.; Bard, A.J. Improved Photoelectrochemical Water Oxidation by the WO3/CuWO4 Composite with a Manganese Phosphate Electrocatalyst. Langmuir 2015, 31, 10897–10903. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Bai, J.; Li, J.; Zeng, Q.; Li, L.; Zhou, B. High-performance BiVO4 photoanodes cocatalyzed with an ultrathin α-Fe2O3 layer for photoelectrochemical application. Appl. Catal. B Environ. 2017, 204, 127–133. [Google Scholar] [CrossRef]
- Pilli, S.K.; Deutsch, T.G.; Furtak, T.E.; Brown, L.D.; Turner, J.A.; Herring, A.M. BiVO(4)/CuWO(4) heterojunction photoanodes for efficient solar driven water oxidation. Phys. Chem. Chem. Phys. 2013, 15, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Guo, M.; Zhang, M. Hydrothermal preparation and optical properties of orientation-controlled WO3 nanorod arrays on ITO substrates. CrystEngComm 2013, 15, 277–284. [Google Scholar] [CrossRef]
- Kanan, M.W.; Yano, J.; Surendranath, Y.; Dincă, M.; Yachandra, V.K.; Nocera, D.G. Structure and Valency of a Cobalt−Phosphate Water Oxidation Catalyst Determined by in Situ X-ray Spectroscopy. J. Am. Chem. Soc. 2010, 132, 13692–13701. [Google Scholar] [CrossRef] [PubMed]
- Symes, M.D.; Surendranath, Y.; Lutterman, D.A.; Nocera, D.G. Bidirectional and Unidirectional PCET in a Molecular Model of a Cobalt-Based Oxygen-Evolving Catalyst. J. Am. Chem. Soc. 2011, 133, 5174–5177. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; Xia, M.; Li, C.; Yue, C.; Diao, P. Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting. Catalysts 2018, 8, 663. https://doi.org/10.3390/catal8120663
Peng B, Xia M, Li C, Yue C, Diao P. Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting. Catalysts. 2018; 8(12):663. https://doi.org/10.3390/catal8120663
Chicago/Turabian StylePeng, Ben, Mengyang Xia, Chao Li, Changshen Yue, and Peng Diao. 2018. "Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting" Catalysts 8, no. 12: 663. https://doi.org/10.3390/catal8120663
APA StylePeng, B., Xia, M., Li, C., Yue, C., & Diao, P. (2018). Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting. Catalysts, 8(12), 663. https://doi.org/10.3390/catal8120663