Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction
Abstract
:1. Introduction
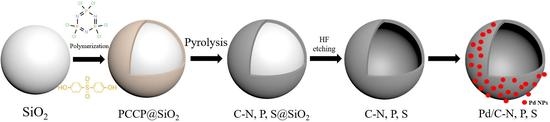
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of C–N,P,S
3.3. Preparation of Pd/C–N,P,S Catalysts
3.4. Electrocatalytic Activity Test
3.5. Catalysts Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Fernandez, P.S.; Lima, F.D.; Troiani, H.E.; Martins, M.E.; Arenillas, A.; Maia, G.; Camara, G.A. Remarkable electrochemical stability of one-step synthesized Pd nanoparticles supported on grapheme and multi-walled carbon nanotubes. Nano Energy 2014, 9, 142–151. [Google Scholar] [CrossRef]
- Dutta, A.; Ouyang, J.Y. Ternary NiAuPt nanoparticles on reduced graphene oxide as catalysts toward the electrochemical oxidation reaction of ethanol. ACS Catal. 2015, 5, 1371–1380. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Sergent, N.; Berthome, G.; Chatenet, M. Huge instability of Pt/C catalysts in alkaline medium. ACS Catal. 2015, 5, 4819–4824. [Google Scholar] [CrossRef]
- Sneed, B.T.; Young, A.P.; Jalalpoor, D.; Golden, M.C.; Mao, S.; Jiang, Y.; Wang, Y.; Tsung, C.K. Shaped Pd-Ni-Pt Core-Sandwich-Shell Nanoparticles: Influence of Ni Sandwich Layers on Catalytic Electrooxidation. ACS Nano 2014, 7, 7239–7250. [Google Scholar] [CrossRef]
- Wang, E.D.; Xu, J.B.; Zhao, T.S. Density Functional Theory Studies of the Structure Sensitivity of Ethanol Oxidation on Palladium Surfaces. J. Phys. Chem. C 2010, 114, 10489–10497. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, F.; Yang, Y.; Cai, W. Carbon supported Pd-Ni-P nanoalloy as an efficient catalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2013, 243, 369–373. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S.W. Highly Active Graphene-Supported NixPd100–x Binary Alloyed Catalysts for Electro-Oxidation of Ethanol in an Alkaline Media. ACS Catal. 2014, 4, 1830–1837. [Google Scholar] [CrossRef]
- Wang, L.; Lavacchi, A.; Bevilacqua, M.; Bellini, M.; Fornasiero, P.; Filippi, J.; Innocenti, M.; Marchionni, A.; Miller, H.A.; Vizza, F. Energy efficiency of alkaline direct ethanol fuel cells employing nanostructured palladium electrocatalysts. ChemCatChem 2015, 7, 2214–2221. [Google Scholar] [CrossRef]
- Singh, R.N.; Awasthi, R. Graphene support for enhanced electrocatalytic activity of Pd for alcohol oxidation. Catal. Sci. Technol. 2011, 1, 778–783. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Q.; Fan, J.C.; Liao, K.X.; Xie, J.W.; Liu, P.; Chen, Y.H.; Min, Y.L.; Xu, Q.J. Highly dispersed palladium nanoparticles on poly (N1, N3-dimethylbenzimidazolium) iodide-functionalized multiwalled carbon nanotubes for ethanol oxidation in alkaline solution. RSC Adv. 2016, 6, 102582–102594. [Google Scholar] [CrossRef]
- Chang, J.F.; Feng, L.G.; Liu, C.P.; Xing, W.; Hu, X.L. An effective Pd–Ni2P/C anode catalyst for direct formic acid fuel cells. Angew. Chem. Int. Ed. 2014, 53, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ostrom, C. Palladium-based nanomaterials: Synthesis and electrochemical applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Meng, H.; Shi, L. Synthesis of mesoporous hollow carbon hemispheres as highly efficient Pd electrocatalyst support for ethanol oxidation. Electrochem. Commun. 2010, 12, 689–692. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, L.; Wang, H. Hollow graphitized carbon nanocage supported Pd catalyst with excellent electrocatalytic activity for ethanol oxidation. ACS Sustain. Chem. Eng. 2018, 6, 7507–7514. [Google Scholar] [CrossRef]
- Kim, A.; Bae, H.S.; Park, J.C.; Song, H. Surfactant-free pd@pSiO2 yolk-shell nanocatalyst for selective oxidation of primary alcohols to aldehydes. New J. Chem. 2015, 39, 8153–8157. [Google Scholar] [CrossRef]
- Jinwoo, K.; Aram, K.; Nallal, M.; Kang, P. PdO/ZnO@mSiO2 hybrid nanocatalyst for reduction of nitroarenes. Catalysts 2018, 8, 280. [Google Scholar]
- Aram, K.; Nallal, M.; Chohye, Y.; Sang, J.; Kang, P. MOF-derived Cu@Cu2O nanocatalyst for oxygen reduction reaction and cycloaddition reaction. Nanomaterials 2018, 8, 138. [Google Scholar]
- Choi, C.H.; Park, S.H.; Woo, S.I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 2012, 8, 7084–7091. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jiang, Z.J.; Maiyalagan, T.; Manthiram, A. Cobalt oxide-coated N- and B-doped graphene hollow spheres as a bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 5877–5889. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; He, S.; Tjiu, W.W.; Pan, J.; Xia, Y.; Liu, T. Nitrogen-doped graphene nanoribbons as efficient metal-free electrocatalysts for oxygen reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Zhang, T.; Silva, T.L.; Almeida, V.C.; Asefa, T. Bone char-derived metal-free N- and S-co-doped nanoporous carbon and its efficient electrocatalytic activity for hydrazine oxidation. Appl. Catal. B 2018, 225, 30–39. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Park, S.H.; Woo, S.I. Additional doping of phosphorus and or sulfur into nitrogen-doped carbon for efficient oxygen reduction reaction in acidic media. Phys. Chem. Chem. Phys. 2013, 15, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fan, J.C.; Min, Y.L.; Wu, T.; Lin, Y.; Xu, Q.J. B, N-codoped graphene nanoribbons supported Pd nanoparticles for ethanol electrooxidation enhancement. J. Mater. Chem. A 2016, 4, 4929–4933. [Google Scholar] [CrossRef]
- Sanchez, M.L.; Primo, A.; Garcia, H. P-doped graphene obtained by pyrolysis of modified alginate as a photocatalyst for hydrogen generation from water–methanol mixtures. Angew. Chem. 2013, 52, 11813–11816. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Park, S.H.; Woo, S.I. N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694–3699. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.; Zhang, L.; Liang, H.W.; Chen, P.; Yu, S.H. N-, P- and Fe-tridoped nanoporous carbon derived from plant biomass: An excellent oxygen reduction electrocatalyst for zinc-air battery. J. Mater. Chem. A 2016, 4, 8602–8609. [Google Scholar]
- Zhang, G.G.; Zang, S.H.; Wang, X.C. Layered Co(OH)2 deposited polymeric carbon nitrides for photocatalytic water oxidation. ACS Catal. 2015, 5, 941–947. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Nasir, M.; Yin, H.; Liu, F.; Hou, Y.L. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Preparation of carbon supported Pd–P catalyst with high content of element phosphorus and its electrocatalytic performance for formic acid oxidation. Electrochem. Commun. 2010, 12, 492–495. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.X.; Chandra, S.T.; Ma, Z.Y.; Huang, H.J.; Zhang, J.F.; Lu, Z.Y.; Huang, W.; Wu, Y.P. Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Interfaces 2016, 8, 10858–10865. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.W.; Xu, Q.; Chen, J.F.; Chen, Z.M.; Huang, X.B.; Tang, X.Z. Controlled fabrication of uniform hollow core porous shell carbon spheres by the pyrolysis of core/shell polystyrene/cross-linked polyphosphazene composites. Chem. Commun. 2010, 46, 6563–6565. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Kim, Y.; Wi, D.H.; Lee, S.; Lee, S.-U.; Lee, Y.W.; Choi, S.-I.; Han, S.W. Ultrathin Free-Standing Ternary-Alloy Nanosheets. Angew. Chem. Int. Ed. 2016, 55, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, P.; Li, Y.; Xia, H.; Wang, D.; Tao, X. Synthesis of open-mouthed, yolk–shell Au@AgPd nanoparticles with access to interior surfaces for enhanced electrocatalysis. Chem. Sci. 2015, 6, 4350–4357. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.-H.; Feng, J.-X.; Li, G.-R. Pd Nanoparticle/CoP Nanosheet Hybrids: Highly Electroactive and Durable Catalysts for Ethanol Electrooxidation. ACS Catal. 2016, 6, 7962–7969. [Google Scholar] [CrossRef]
- Zhang, K.; Bin, D.; Yang, B.; Wang, C.; Ren, F.; Du, Y. Ru-assisted synthesis of Pd/Ru nanodendrites with high activity for ethanol electrooxidation. Nanoscale 2015, 7, 12445–12451. [Google Scholar] [CrossRef]
- Wang, A.-L.; He, X.-J.; Lu, X.-F.; Xu, H.; Tong, Y.-X.; Li, G.-R. Palladium–Cobalt Nanotube Arrays Supported on Carbon Fiber Cloth as High-Performance Flexible Electrocatalysts for Ethanol Oxidation. Angew. Chem. Int. Ed. 2015, 54, 3669–3673. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.; Tang, D.; Zhang, T.; Wang, Y. Ethanol oxidation on Pd/C promoted with CaSiO3 in alkaline medium. Electrochim. Acta 2015, 158, 18–23. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.; Huang, G.; Yang, N.; Zhang, L.; Wang, H.; Liu, Y.; Wang, W.; Gao, J. A green method to prepare Pd–Ag nanoparticles supported on reduced graphene oxide and their electrochemical catalysis of methanol and ethanol oxidation. J. Power Sources 2014, 263, 13–21. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Lu, G.Q.; Kenis, P.J.A.; Wieckowski, A. Electrooxidation of adsorbed CO on Pt(111) and Pt(111)/Ru in alkaline media and comparison with results from acidic media. J. Electroanal. Chem. 2004, 568, 215–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Tian, J.; Wang, F.; Zhan, L. A facile and novel approach toward synthetic polypyrrole oligomers functionalization of multi-walled carbon nanotubes as PtRu catalyst support for methanol electro-oxidation. J. Power Sources 2010, 195, 4634–4640. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Lin, Y.; Fan, J.; Li, Q.; Shi, P.; Xu, Q.; Min, Y. Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts 2019, 9, 114. https://doi.org/10.3390/catal9020114
Yu K, Lin Y, Fan J, Li Q, Shi P, Xu Q, Min Y. Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts. 2019; 9(2):114. https://doi.org/10.3390/catal9020114
Chicago/Turabian StyleYu, Ke, Yan Lin, Jinchen Fan, Qiaoxia Li, Penghui Shi, Qunjie Xu, and Yulin Min. 2019. "Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction" Catalysts 9, no. 2: 114. https://doi.org/10.3390/catal9020114
APA StyleYu, K., Lin, Y., Fan, J., Li, Q., Shi, P., Xu, Q., & Min, Y. (2019). Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts, 9(2), 114. https://doi.org/10.3390/catal9020114