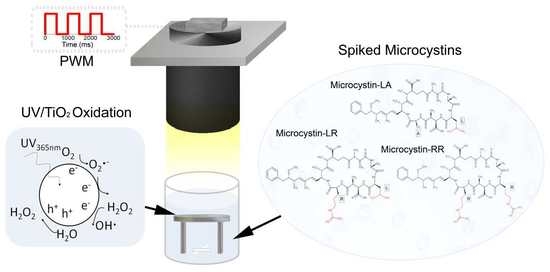
Photocatalytic Degradation of Microcystins by TiO2 Using UV-LED Controlled Periodic Illumination
Abstract
:1. Introduction
2. Results and Discussion
2.1. PTT Membrane Characterization
2.2. TPA Conversion
2.3. Degradation of Microcystins under Continuous Light
2.4. Degradation of Microcystins under CPI
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. PTT Membrane Synthesis and Characterization
3.3. Experimental Setup for Microcystin Degradation
3.4. Experimental Setup for TPA Conversion
3.5. Electrical Energy per Order
3.6. Sample Preparation and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dawson, R.M. The Toxicology of Microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umehara, A.; Takahashi, T.; Komorita, T.; Orita, R.; Chio, J.-W.; Takenaka, R.; Mabuchi, R.; Park, H.-D.; Tsutsumi, H. Widespread dispersal and bio-accumulation of toxic microcystins in benthic marine ecosystems. Chemoshpere 2017, 167, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P.; Lund, U.; Galal-Gorchev, H.; Ohanian, E. Guidelines for Drinking-Water Quality Volume 2— Health Criteria and Other Supporting Information, 2nd ed.; Kenny, J., Galal-Gorchev, H., Eds.; World Health Organisation: Geneva, Switzerland, 1998; Volume 2. [Google Scholar]
- Committee on Drinking Water. Cyanobacterial Toxins in Drinking Water; Committee on Drinking Water: Ottawa, ON, Canada, 2016. [Google Scholar]
- Wolf, D.; Klaiber, H.A. Bloom and bust: Toxic algae’s impact on nearby property values. Ecol. Econ. 2017, 135, 209–221. [Google Scholar] [CrossRef]
- Dyson, K.; Huppert, D.D. Regional economic impacts of razor clam beach closures due to harmful algal blooms (HABs) on the Pacific coast of Washington. Harmful Algae 2010, 9, 264–271. [Google Scholar] [CrossRef]
- Sharma, V.K.; Triantis, T.M.; Antoniou, M.G.; He, X.; Pelaez, M.; Han, C.; Song, W.; O’shea, K.E.; De La Cruz, A.A.; Kaloudis, T.; et al. Destruction of microcystins by conventional and advanced oxidation processes: A review. Sep. Purif. Technol. 2012, 91, 3–17. [Google Scholar] [CrossRef]
- Liu, I.; Lawton, L.A.; Bahnemann, D.W.; Liu, L.; Proft, B.; Robertson, P.K.J. The photocatalytic decomposition of microcystin-LR using selected titanium dioxide materials. Chemosphere 2009, 76, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Om, S.S.O.; De Villiers, D.; Engelbrecht, W.J.; E El, G.; Wessels, F.S. Degradation of microcystin toxins in a falling film photocatalytic reactor with immobilized titanium dioxide catalyst. Water Res. 2002, 36, 140–146. [Google Scholar] [CrossRef]
- Cornish, B.J.P.A.; Lawton, L.A.; Robertson, P.K.J. Hydrogen peroxide enhanced photocatalytic oxidation of microcystin-LR using titanium dioxide. Appl. Catal. B Environ. 2000, 25, 59–67. [Google Scholar] [CrossRef]
- Lawton, L.A.; Robertson, P.K.J.; Cornish, B.J.P.A.; Marr, I.L.; Jaspars, M. Processes influencing surface interaction and photocatalytic destruction of microcystins on titanium dioxide photocatalysts. J. Catal. 2003, 213, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, L.; Meric, S.; Guida, M.; Kassinos, D.; Belgiorno, V. Heterogenous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res. 2009, 43, 4070–4078. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Hu, A.; Li, W.; Zhou, Y.N. Enhanced degradation of persistent pharmaceuticals found in wastewater treatment effluents using TiO2 nanobelt photocatalysts. J. Nanopart. Res. 2013, 15, 1990. [Google Scholar] [CrossRef]
- Martínez, C.; Canle L., M.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Catal. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Arlos, M.J.; Liang, R.; Hatat-Fraile, M.M.; Bragg, L.M.; Zhou, N.Y.; Servos, M.R.; Andrews, S.A. Photocatalytic decomposition of selected estrogens and their estrogenic activity by UV-LED irradiated TiO2 immobilized on porous titanium sheets via thermal-chemical oxidation. J. Hazard. Mater. 2016, 318, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Arlos, M.J.; Hatat-Fraile, M.M.; Liang, R.; Bragg, L.M.; Zhou, N.Y.; Andrews, S.A.; Servos, M.R. Photocatalytic decomposition of organic micropollutants using immobilized TiO2 having different isoelectric points. Water Res. 2016, 101, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Shiu, S.-J.; Wu, H.-C. Decomposition of dimethyl phthalate in aqueous solution by UV–LED/TiO2 process under periodic illumination. J. Photochem. Photobiol. A Chem. 2017, 332, 299–305. [Google Scholar] [CrossRef]
- Buechler, K.J.; Nam, C.H.; Zawistowski, T.M.; Noble, R.D.; Koval, C.A. Design and Evaluation of a Novel-Controlled Periodic Illumination Reactor To Study Photocatalysis. Ind. Eng. Chem. Res. 1999, 38, 1258–1263. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Hegedűs, P.; Szabó-Bárdos, E.; Horváth, O.; Szabó, P.; Horváth, K. Investigation of a TiO2 photocatalyst immobilized with poly(vinyl alcohol). Catal. Today 2017, 284, 179–186. [Google Scholar] [CrossRef]
- Sczechowski, J.G.; Koval, C.A.; Noble, R.D. Evidence of critical illumination and dark recovery times for increasing the photoefficiency of aqueous heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 1993, 74, 273–278. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. Controlled periodic illumination in semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 2016, 319–320, 96–106. [Google Scholar] [CrossRef]
- Liang, R.; Van Leuwen, J.C.; Bragg, L.M.; Arlos, M.J.; Li Chun Fong, L.C.M.; Schneider, O.M.; Peng, P.; Servos, M.R.; Zhou, Y.N. Utilizing UV-LED pulse width modulation on TiO2 advanced oxidation processes to enhance the decomposition efficiency of pharmaceutical micropollutants. Chem. Eng. J. 2019, 361, 439–449. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. Environmental TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Memming, R. Photoinduced charge transfer processes at semiconductor electrodes and particles. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1994; Volume 169, pp. 105–181. ISBN 978-3-540-57565-8. [Google Scholar]
- Sczechowski, J.G.; Koval, C.A.; Noble, R.D. A Taylor vortex reactor for heterogeneous photocatalysis. Chem. Eng. Sci. 1995, 50, 3163–3173. [Google Scholar] [CrossRef]
- Miranda-García, N.; Maldonado, M.I.; Coronado, J.M.; Malato, S. Degradation study of 15 emerging contaminants at low concentration by immobilized TiO2 in a pilot plant. Catal. Today 2010, 151, 107–113. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Mai, J.; Ni, J. Initial photocatalytic degradation intermediates/pathways of 17α-ethynylestradiol: Effect of pH and methanol. Chemosphere 2010, 81, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nasuhoglu, D.; Berk, D.; Yargeau, V. Photocatalytic removal of 17α-ethinylestradiol (EE2) and levonorgestrel (LNG) from contraceptive pill manufacturing plant wastewater under UVC radiation. Chem. Eng. J. 2012, 185–186, 52–60. [Google Scholar] [CrossRef]
- Kralchevska, R.; Milanova, M.; Bistan, M.; Pintar, A.; Todorovsky, D. The photocatalytic degradation of 17α-ethynylestradiol by pure and carbon nanotubes modified TiO2 under UVC illumination. Open Chem. 2012, 10, 1137–1148. [Google Scholar] [CrossRef]
- Marinho, B.A.; de Liz, M.V.; Lopes Tiburtius, E.R.; Nagata, N.; Peralta-Zamora, P.; Iguchi, T.; Kubota, Y.; Fujishima, A. TiO2 and ZnO mediated photocatalytic degradation of E2 and EE2 estrogens. Photochem. Photobiol. Sci. 2013, 12, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.L.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of pharmaceuticals and endocrine disrupting chemicals by a submerged membrane photocatalysis reactor (MPR). Sep. Purif. Technol. 2014, 127, 131–139. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Photocatalysis and Water Purification: From Fundamentals to Recent Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 3–23. [Google Scholar]
- Paul, T.; Miller, P.L.; Strathmann, T.J. Visible-light-mediated TiO2 photocatalysis of fluoroquinolone antibacterial agents. Environ. Sci. Technol. 2007, 41, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Cernigoj, U.; Kete, M.; Stangar, U.L. Development of a fluorescence-based method for evaluation of self-cleaning properties of photocatalytic layers. Catal. Today 2010, 151, 46. [Google Scholar] [CrossRef]
- Gora, S.; Liang, R.; Zhou, Y.N.; Andrews, S. Settleable engineered titanium dioxide nanomaterials for the removal of natural organic matter from drinking water. Chem. Eng. J. 2018, 334, 638–649. [Google Scholar] [CrossRef]
- Liang, R.; Li Chun Fong, L.C.M.; Arlos, M.J.; Van Leeuwen, J.; Shahnam, E.; Peng, P.; Servos, M.R.; Zhou, Y.N. Photocatalytic degradation using one-dimensional TiO2 and Ag-TiO2 nanobelts under UV-LED controlled periodic illumination. J. Environ. Chem. Eng. 2017, 5, 4365–4373. [Google Scholar] [CrossRef]



| Compound | Charge at pH 5a |
|---|---|
| MC-LA | −1.9332 |
| MC-LR | −0.9329 |
| MC-RR | 0.0567 |
| Q1a (Da) | Q3b (Da) | Time (ms) | DPc (volts) | EPd (volts) | CEe (volts) | CXPf (volts) | CEPg (volts) | Retention Time (min) | |
|---|---|---|---|---|---|---|---|---|---|
| NOD | 825.563 | 135.3 | 150 | 96 | 12 | 75 | 4 | 40 | 4.55 |
| MC-LA | 911.395 | 135.2 | 150 | 51 | 12 | 81 | 4 | 36 | 4.62 |
| MC-LR | 995.699 | 135.1 | 150 | 116 | 12 | 99 | 4 | 36 | 4.67 |
| MC-RR | 519.960 | 135.2 | 150 | 131 | 7 | 41 | 4 | 26 | 4.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, O.M.; Liang, R.; Bragg, L.; Jaciw-Zurakowsky, I.; Fattahi, A.; Rathod, S.; Peng, P.; Servos, M.R.; Zhou, Y.N. Photocatalytic Degradation of Microcystins by TiO2 Using UV-LED Controlled Periodic Illumination. Catalysts 2019, 9, 181. https://doi.org/10.3390/catal9020181
Schneider OM, Liang R, Bragg L, Jaciw-Zurakowsky I, Fattahi A, Rathod S, Peng P, Servos MR, Zhou YN. Photocatalytic Degradation of Microcystins by TiO2 Using UV-LED Controlled Periodic Illumination. Catalysts. 2019; 9(2):181. https://doi.org/10.3390/catal9020181
Chicago/Turabian StyleSchneider, Olivia M., Robert Liang, Leslie Bragg, Ivana Jaciw-Zurakowsky, Azar Fattahi, Shasvat Rathod, Peng Peng, Mark R. Servos, and Y. Norman Zhou. 2019. "Photocatalytic Degradation of Microcystins by TiO2 Using UV-LED Controlled Periodic Illumination" Catalysts 9, no. 2: 181. https://doi.org/10.3390/catal9020181
APA StyleSchneider, O. M., Liang, R., Bragg, L., Jaciw-Zurakowsky, I., Fattahi, A., Rathod, S., Peng, P., Servos, M. R., & Zhou, Y. N. (2019). Photocatalytic Degradation of Microcystins by TiO2 Using UV-LED Controlled Periodic Illumination. Catalysts, 9(2), 181. https://doi.org/10.3390/catal9020181