Heterogeneous Fenton-Like Degradation of p-Nitrophenol over Tailored Carbon-Based Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Carbon-Based Materials Characterization
2.2. Adsorption in Carbon-Based Materials
2.3. Catalytic Wet Peroxidation (CWPO) Using the Supports as Catalysts
2.4. Fenton Reaction
2.5. Supports and Catalysts Stability and Reusability
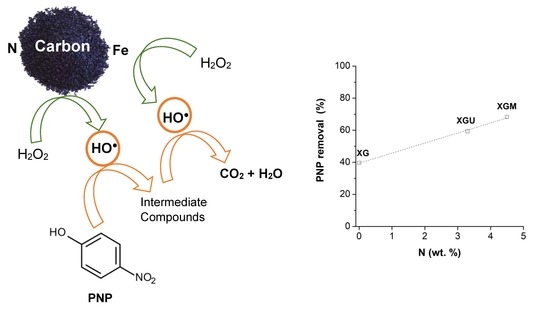
2.6. Effect of Nitrogen Content
2.7. Evaluation of Intermediates Compounds Formed during the Heterogeneous Fenton Reaction
3. Materials and Methods
3.1. Materials
3.2. Carbon-Based Materials Preparation and Characterization
3.3. Adsorption and Reaction Tests
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Sonntag, C. Advanced oxidation processes: Mechanistic aspects. Water Sci. Technol. 2008, 58, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S. Use of selected advanced oxidation processes (aops) for wastewater treatment—A mini review. Glob. NEST J. 2008, 10, 376–385. [Google Scholar]
- Laherty, K.A.; Huang, C.P. Continuous flow applications of fenton’s reagent for the treatment of reafractory wastewater. In Proceedings of the Second International Symposium on Chemical Oxidation-Technologies for the Nineties, Nashville, TN, USA, 19–21 February 1992; CRC Press: Nashville, TN, USA, 1992. [Google Scholar]
- Bigda, R.J. Consider fenton’s chemistry for wastewater treatment. Chem. Eng. Prog. 1995, 91, 62–66. [Google Scholar]
- Arslan, İ.; Akmehmet Balcioǧlu, I.; Tuhkanen, T. Oxidative treatment of simulated dyehouse effluent by uv and near-uv light assisted fenton’s reagent. Chemosphere 1999, 39, 2767–2783. [Google Scholar] [CrossRef]
- Papadopoulos, A.E.; Fatta, D.; Loizidou, M. Development and optimization of dark fenton oxidation for the treatment of textile wastewaters with high organic load. J. Hazard. Mater. 2007, 146, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Queirós, S.; Morais, V.; Rodrigues, C.S.D.; Maldonado-Hódar, F.J.; Madeira, L.M. Heterogeneous fenton’s oxidation using fe/zsm-5 as catalyst in a continuous stirred tank reactor. Sep. Purif. Technol. 2015, 141, 235–245. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Heterogeneous fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Herney-Ramirez, J.; Vicente, M.A.; Madeira, L.M. Heterogeneous photo-fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review. Appl. Catal. B Environ. 2010, 98, 10–26. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Sashkina, K.A.; Polukhin, A.V.; Labko, V.S.; Ayupov, A.B.; Lysikov, A.I.; Parkhomchuk, E.V. Fe-silicalites as heterogeneous fenton-type catalysts for radiocobalt removal from edta chelates. Appl. Catal. B Environ. 2016, 185, 353–361. [Google Scholar] [CrossRef]
- Esteves, B.M.; Rodrigues, C.S.D.; Boaventura, R.A.R.; Maldonado-Hódar, F.J.; Madeira, L.M. Coupling of acrylic dyeing wastewater treatment by heterogeneous fenton oxidation in a continuous stirred tank reactor with biological degradation in a sequential batch reactor. J. Environ. Manag. 2016, 166, 193–203. [Google Scholar] [CrossRef]
- Duarte, F.; Maldonado-Hódar, F.J.; Madeira, L.M. New insight about orange ii elimination by characterization of spent activated carbon/fe fenton-like catalysts. Appl. Catal. B Environ. 2013, 129, 264–272. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Phillips, J.; Xia, B.; Radovic, L.R. On the modification and characterization of chemical surface properties of activated carbon: In the search of carbons with stable basic properties. Langmuir 1996, 12, 4404–4410. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Soares, O.S.G.P.; Pinho, M.T.; Pereira, M.F.R.; Madeira, L.M. P-nitrophenol degradation by heterogeneous fenton’s oxidation over activated carbon-based catalysts. Appl. Catal. B Environ. 2017, 219, 109–122. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Lin, A.; Guo, Z. Effective removal of phenol by using activated carbon supported iron prepared under microwave irradiation as a reusable heterogeneous fenton-like catalyst. J. Environ. Chem. Eng. 2017, 5, 870–876. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Moreno-Castilla, C.; Costa, C.A.; Madeira, L.M. Azo-dye orange ii degradation by heterogeneous fenton-like reaction using carbon-fe catalysts. Appl. Catal. B Environ. 2007, 75, 312–323. [Google Scholar] [CrossRef]
- Messele, S.A.; Soares, O.S.G.P.; Órfão, J.J.M.; Bengoa, C.; Stüber, F.; Fortuny, A.; Fabregat, A.; Font, J. Effect of activated carbon surface chemistry on the activity of zvi/ac catalysts for fenton-like oxidation of phenol. Catal. Today 2015, 240, 73–79. [Google Scholar] [CrossRef]
- Cleveland, V.; Bingham, J.-P.; Kan, E. Heterogeneous fenton degradation of bisphenol a by carbon nanotube-supported Fe3O4. Sep. Purif. Technol. 2014, 133, 388–395. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Lian, C.; Wei, F.; Zhang, D.; Wu, G.; Chen, B.; Wang, S. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as fenton-like catalysts for organic pollutant removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.K.; Mousavinia, F.; Khalafi-Nezhad, A.; Firouzabadi, H.; Gil, A. Degradation of methyl orange by heterogeneous fenton-like oxidation on a nano-organometallic compound in the presence of multi-walled carbon nanotubes. Chem. Eng. Res. Des. 2016, 112, 113–121. [Google Scholar] [CrossRef]
- Carrasco-Díaz, M.R.; Castillejos-López, E.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. On the textural and crystalline properties of fe-carbon xerogels. Application as fenton-like catalysts in the oxidation of paracetamol by H2O2. Microporous Mesoporous Mater. 2017, 237, 282–293. [Google Scholar] [CrossRef]
- Pinho, M.T.; Gomes, H.T.; Ribeiro, R.S.; Faria, J.L.; Silva, A.M.T. Carbon nanotubes as catalysts for catalytic wet peroxide oxidation of highly concentrated phenol solutions: Towards process intensification. Appl. Catal. B Environ. 2015, 165, 706–714. [Google Scholar] [CrossRef]
- Alegre, C.; Calvillo, L.; Moliner, R.; González-Expósito, J.A.; Guillén-Villafuerte, O.; Huerta, M.V.M.; Pastor, E.; Lázaro, M.J. Pt and ptru electrocatalysts supported on carbon xerogels for direct methanol fuel cells. J. Power Sources 2011, 196, 4226–4235. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Nitrophenols: 2-Nitrophenol and 4-Nitrophenol; Public Agency for Toxic Substances and Disease Registry, Health Service: Atlanta, GA, USA, 1992.
- Tang, L.; Tang, J.; Zeng, G.; Yang, G.; Xie, X.; Zhou, Y.; Pang, Y.; Fang, Y.; Wang, J.; Xiong, W. Rapid reductive degradation of aqueous p-nitrophenol using nanoscale zero-valent iron particles immobilized on mesoporous silica with enhanced antioxidation effect. Appl. Surf. Sci. 2015, 333, 220–228. [Google Scholar] [CrossRef]
- Ji, Q.; Li, J.; Xiong, Z.; Lai, B. Enhanced reactivity of microscale Fe/Cu bimetallic particles (Mfe/Cu) with persulfate (ps) for p-nitrophenol (pnp) removal in aqueous solution. Chemosphere 2017, 172, 10–20. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, N.; Zhou, J.; Jiang, P.; Liu, G. Heterogeneous fenton-like catalytic removal of p-nitrophenol in water using acid-activated fly ash. J. Hazard. Mater. 2012, 201–202, 68–73. [Google Scholar] [CrossRef]
- Subbulekshmi, N.L.; Subramanian, E. Nano CuO immobilized fly ash zeolite fenton-like catalyst for oxidative degradation of p-nitrophenol and p-nitroaniline. J. Environ. Chem. Eng. 2017, 5, 1360–1371. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, X.; He, Z.; Tan, W.; Zhu, J.; Yuan, P.; Zhu, R.; He, H. The constraints of transition metal substitutions (Ti, Cr, Mn, Co and Ni) in magnetite on its catalytic activity in heterogeneous fenton and uv/fenton reaction: From the perspective of hydroxyl radical generation. Appl. Catal. B Environ. 2014, 150–151, 612–618. [Google Scholar] [CrossRef]
- Ferroudj, N.; Nzimoto, J.; Davidson, A.; Talbot, D.; Briot, E.; Dupuis, V.; Bée, A.; Medjram, M.S.; Abramson, S. Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic fenton catalysts for the removal of water pollutants. Appl. Catal. B Environ. 2013, 136–137, 9–18. [Google Scholar] [CrossRef]
- Wan, D.; Li, W.; Wang, G.; Lu, L.; Wei, X. Degradation of p-nitrophenol using magnetic fe0/fe3o4/coke composite as a heterogeneous fenton-like catalyst. Sci. Total Environ. 2017, 574, 1326–1334. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Removal of 2-nitrophenol by catalytic wet peroxide oxidation using carbon materials with different morphological and chemical properties. Appl. Catal. B Environ. 2013, 140, 356–362. [Google Scholar] [CrossRef]
- Messele, S.A.; Soares, O.S.G.P.; Órfão, J.J.M.; Bengoa, C.; Font, J. Zero-valent iron supported on nitrogen-doped carbon xerogel as catalysts for the oxidation of phenol by fenton-like system. Environ. Technol. 2018, 39, 2951–2958. [Google Scholar] [CrossRef]
- Dhaouadi, A.; Adhoum, N. Heterogeneous catalytic wet peroxide oxidation of paraquat in the presence of modified activated carbon. Appl. Catal. B Environ. 2010, 97, 227–235. [Google Scholar] [CrossRef]
- Messele, S.A.; Soares, O.S.G.P.; Órfão, J.J.M.; Stüber, F.; Bengoa, C.; Fortuny, A.; Fabregat, A.; Font, J. Zero-valent iron supported on nitrogen-containing activated carbon for catalytic wet peroxide oxidation of phenol. Appl. Catal. B Environ. 2014, 154, 329–338. [Google Scholar] [CrossRef]
- Yang, G.; Chen, H.; Qin, H.; Feng, Y. Amination of activated carbon for enhancing phenol adsorption: Effect of nitrogen-containing functional groups. Appl. Surf. Sci. 2014, 293, 299–305. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Faria, P.C.C.; Órfão, J.J.M. Decolourisation of dye solutions by oxidation with H2O2 in the presence of modified activated carbons. J. Hazard. Mater. 2009, 162, 736–742. [Google Scholar] [CrossRef]
- Sun, H.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tadé, M.O.; Wang, S. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154, 134–141. [Google Scholar] [CrossRef]
- Santos, D.F.M.; Soares, O.S.G.P.; Silva, A.M.T.; Figueiredo, J.L.; Pereira, M.F.R. Catalytic wet oxidation of organic compounds over n-doped carbon nanotubes in batch and continuous operation. Appl. Catal. B Environ. 2016, 199, 361–371. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sun, B.; Zhu, X. Aqueous 4-nitrophenol decomposition and hydrogen peroxide formation induced by contact glow discharge electrolysis. J. Hazard. Mater. 2010, 181, 1010–1015. [Google Scholar] [CrossRef]
- Sun, S.-P.; Lemley, A.T. P-nitrophenol degradation by a heterogeneous fenton-like reaction on nano-magnetite: Process optimization, kinetics, and degradation pathways. J. Mol. Catal. A Chem. 2011, 349, 71–79. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Borges, R.A.C.; Lima, V.N.; Madeira, L.M. P-nitrophenol degradation by fenton’s oxidation in a bubble column reactor. J. Environ. Manag. 2018, 206, 774–785. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Liu, P.; Yu, Y.; Zhao, Y.; Li, X.; Jiang, W.; Wang, J.; Yang, X.; Sun, C. Effect of structural defects on activated carbon catalysts in catalytic wet peroxide oxidation of m-cresol. Catal. Today 2015, 258, 120–131. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Rodríguez, J.J. Catalytic wet peroxide oxidation of phenol with a fe/active carbon catalyst. Appl. Catal. B Environ. 2006, 65, 261–268. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Fathy, N.A.; Attia, A.A.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Activated carbon xerogels for the removal of the anionic azo dyes orange ii and chromotrope 2r by adsorption and catalytic wet peroxide oxidation. Chem. Eng. J. 2012, 195–196, 112–121. [Google Scholar] [CrossRef]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Silva, A.M.T.; Faria, J.L. Activated carbons treated with sulphuric acid: Catalysts for catalytic wet peroxide oxidation. Catal. Today 2010, 151, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.P.S.; Pereira, M.F.R.; Figueiredo, J.L. Carbon xerogel catalyst for no oxidation. Catalysts 2012, 2, 447. [Google Scholar] [CrossRef]
- Sousa, J.P.S.; Pereira, M.F.R.; Figueiredo, J.L. No oxidation over nitrogen doped carbon xerogels. Appl. Catal. B Environ. 2012, 125, 398–408. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Gallegos-Suarez, E.; Castillejos, E.; Rodriguez-Ramos, I.; Pereira, M.F.R. Nitrate reduction over a pd-cu/mwcnt catalyst: Application to a polluted groundwater. Environ. Technol. 2012, 33, 2353–2358. [Google Scholar] [CrossRef]
- Bhatti, Z.I.; Toda, H.; Furukawa, K. P-nitrophenol degradation by activated sludge attached on nonwovens. Water Res. 2002, 36, 1135–1142. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Pollution Control Federation: Washington, DC, USA, 1998. [Google Scholar]
- Sellers, R.M. Spectrophotometric determination of hydrogen peroxide using potassium titanium (IV) oxalate. Analyst 1980, 105, 950–954. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Gao, J.; Jin, X.; Wang, Z.; Wang, B.; Li, K.; Li, Y. Detection and comparison of reactive oxygen species (Ros) generated by chlorophyllin metal (Fe, Mg and Cu) complexes under ultrasonic and visible-light irradiation. Ultrason. Sonochem. 2011, 18, 1028–1034. [Google Scholar] [CrossRef]









| Sample | SBET ** (±10 m2 g−1) | Smeso ** (±10 m2 g−1) | Vmicro ** (±0.01 cm3 g−1) | Vp P/Po=0.95 ** (cm3 g−1) |
|---|---|---|---|---|
| AC | 824 | 196 | 0.286 | 0.492 |
| ACM | 730 | 174 | 0.229 | 0.408 |
| CNT | 273 | 273 | 0 | 0.573 |
| CNTM | 222 | 222 | 0 | 0.512 |
| XG | 572 | 290 | 0.150 | 0.497 |
| XGM | 510 | 117 | 0.182 | 0.398 |
| XGU | 513 | 174 | 0.158 | 0.486 |
| Fe/AC | 807 | 179 | 0.288 | 0.479 |
| Fe/ACM | 717 | 171 | 0.251 | 0.408 |
| Fe/CNT | 266 | 266 | 0 | 0.540 |
| Fe/CNTM | 205 | 205 | 0 | 0.471 |
| Fe/XG | 564 | 273 | 0.149 | 0.491 |
| Fe/XGM | 490 | 133 | 0.168 | 0.402 |
| Fe/XGU | 486 | 166 | 0.147 | 0.449 |
| Sample | N Content | N-6 ** | N-5 ** | N-Q ** | pHPZC (±0.1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| NEA (wt.%) | NXPS (wt.%) | B.E. (eV) | (wt.%) | B.E. (eV) | (wt.%) | B.E. (eV) | (wt.%) | ||
| AC | 0.15 | - | - | - | - | - | - | - | 7.5 |
| ACM | 3.40 | 3.14 | 398.4 | 1.68 | 400.0 | 1.07 | 401.4 | 0.39 | 7.5 |
| CNT | 0.01 | - | - | - | - | - | - | - | 7.0 |
| CNTM | 5.31 | 4.8 | 398.9 | 2.24 | 400.3 | 1.66 | 401.5 | 0.87 | 7.5 |
| XG | 0 | - | - | - | - | - | - | - | 8.0 |
| XGM | 4.51 | 4.64 | 398.5 | 2.48 | 400.6 | 2.16 | - | - | 8.8 |
| XGU | 3.30 | 3.87 | 398.6 | 2.18 | 400.7 | 1.70 | - | - | 8.3 |
| t (min) | PNP (mgC L−1) | Pyruvic (mgC L−1) | Maleic (mgC L−1) | Oxalic (mgC L−1) | Total Carbon * (mgC L−1) | TOC (mgC L−1) | Contribution ** (%) |
|---|---|---|---|---|---|---|---|
| 0 | 260.7 | 0.0 | 0.0 | 0.0 | 260.7 | 260.9 | 99.9 |
| 5 | 105.3 | 7.3 | 6.4 | 5.1 | 124.1 | 126.3 | 98.3 |
| 10 | 86.3 | 9.0 | 8.6 | 6.3 | 110.1 | 112.4 | 98.0 |
| 15 | 78.0 | 11.0 | 10.9 | 7.5 | 107.5 | 107.3 | 100.2 |
| 30 | 62.1 | 13.9 | 12.8 | 11.0 | 99.9 | 100.8 | 99.1 |
| 45 | 55.6 | 13.0 | 12.0 | 13.9 | 94.5 | 94.6 | 99.9 |
| 60 | 52.7 | 12.5 | 11.8 | 13.1 | 90.1 | 92.8 | 97.1 |
| 120 | 50.9 | 12.4 | 11.7 | 13.0 | 87.4 | 92.2 | 95.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, O.S.G.P.; Rodrigues, C.S.D.; Madeira, L.M.; Pereira, M.F.R. Heterogeneous Fenton-Like Degradation of p-Nitrophenol over Tailored Carbon-Based Materials. Catalysts 2019, 9, 258. https://doi.org/10.3390/catal9030258
Soares OSGP, Rodrigues CSD, Madeira LM, Pereira MFR. Heterogeneous Fenton-Like Degradation of p-Nitrophenol over Tailored Carbon-Based Materials. Catalysts. 2019; 9(3):258. https://doi.org/10.3390/catal9030258
Chicago/Turabian StyleSoares, O. S. G. P., Carmen S. D. Rodrigues, Luis M. Madeira, and M. F. R. Pereira. 2019. "Heterogeneous Fenton-Like Degradation of p-Nitrophenol over Tailored Carbon-Based Materials" Catalysts 9, no. 3: 258. https://doi.org/10.3390/catal9030258
APA StyleSoares, O. S. G. P., Rodrigues, C. S. D., Madeira, L. M., & Pereira, M. F. R. (2019). Heterogeneous Fenton-Like Degradation of p-Nitrophenol over Tailored Carbon-Based Materials. Catalysts, 9(3), 258. https://doi.org/10.3390/catal9030258