Hydrogen Production from Formic Acid over Au Catalysts Supported on Carbon: Comparison with Au Catalysts Supported on SiO2 and Al2O3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electron Microscopy Study
2.2. XPS Study
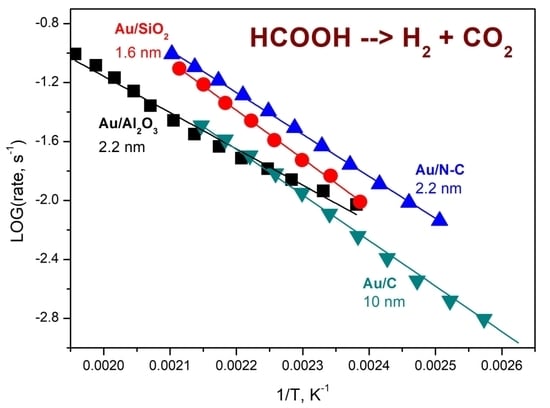
2.3. Catalytic Activity
2.4. DFT Calculations
- >N + HCOOH → >NH+HCOO−
- >NH+HCOO− + Au → >NH + AuH + CO2
- >NH + AuH → >N + Au + H2.
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Catalytic Measurements
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scurrell, M.S. Thoughts on the Use of Gold-Based Catalysts in Environmental Protection Catalysis. Gold Bull. 2017, 50, 77–84. [Google Scholar] [CrossRef]
- Prati, L.; Villa, A.; Lupini, A.R.; Veith, G.M. Gold on Carbon: One Billion Catalysts under a Single Label. Phys. Chem. Chem. Phys. 2012, 14, 2969–2978. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The Role of Carbon Materials in Heterogeneous Catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Yuranov, I.; Suvorova, E.I.; Buffat, P.A.; Kiwi-Minsker, L. Highly Dispersed Gold on Activated Carbon Fibers for Low-Temperature CO Oxidation. J. Catal. 2004, 224, 8–17. [Google Scholar] [CrossRef]
- Pyryaev, P.A.; Moroz, B.L.; Zyuzin, D.A.; Nartova, A.V.; Bukhtiyarov, V.I. Nanosized Au/C Catalyst Obtained from a Tetraamminegold(III) Precursor: Synthesis, Characterization, and Catalytic Activity in Low-Temperature CO Oxidation. Kinet. Catal. 2010, 51, 885–892. [Google Scholar] [CrossRef]
- Megias-Sayago, C.; Santos, J.L.; Ammari, F.; Chenouf, M.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Influence of Gold Particle Size in Au/C Catalysts for Base-Free Oxidation of Glucose. Catal. Today 2018, 306, 183–190. [Google Scholar] [CrossRef]
- Yushchenko, D.Y.; Simonov, P.A.; Khlebnikova, T.B.; Pai, Z.P.; Bukhtiyarov, V.I. Oxidation of N-Isopropyl Phosphonomethyl Glycine with Hydrogen Peroxide Catalyzed by Carbon-Supported Gold Nanoparticles. Catal. Commun. 2019, 121, 57–61. [Google Scholar] [CrossRef]
- Liotta, L.F. New Trends in Gold Catalysts. Catalysts 2014, 4, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Albani, D.; Fako, E.; Kaiser, S.K.; Safonova, O.V.; López, N.; Pérez-Ramírez, J. Design of Single Gold Atoms on Nitrogen-Doped Carbon for Molecular Recognition in Alkyne Semi-Hydrogenation. Angew. Chem. Int. Ed. 2019, 58, 504–509. [Google Scholar] [CrossRef]
- Grad, O.; Mihet, M.; Dan, M.; Blanita, G.; Radu, T.; Berghian-Grosan, C.; Lazar, M.D. Au/Reduced Graphene Oxide Composites: Eco-Friendly Preparation Method and Catalytic Applications for Formic Acid Dehydrogenation. J. Mater. Sci. 2019, 54, 6991–7004. [Google Scholar] [CrossRef]
- Gianotti, E.; Taillades-Jacquin, M.; Roziere, J.; Jones, D.J. High-Purity Hydrogen Generation Via Dehydrogenation of Organic Carriers: A Review on the Catalytic Process. ACS Catal. 2018, 8, 4660–4680. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment Based on Chemical and Economic Properties. Int. J. Hydrog. Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Zhong, H.; Iguchi, M.; Chatterjee, M.; Himeda, Y.; Xu, Q.; Kawanami, H. Formic Acid-Based Liquid Organic Hydrogen Carrier System with Heterogeneous Catalysts. Adv. Sustain. Syst. 2018, 2, 1700161. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef]
- Preuster, P.; Albert, J. Biogenic Formic Acid as a Green Hydrogen Carrier. Energy Technol. 2018, 6, 501–509. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Heterogeneous Catalysts for Hydrogenation of CO2 and Bicarbonates to Formic Acid and Formates. Catal. Rev. 2018, 60, 566–593. [Google Scholar] [CrossRef]
- Wang, W.H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 Hydrogenation to Formate and Methanol as an Alternative to Photo- and Electrochemical CO2 Reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Ojeda, M.; Iglesia, E. Formic Acid Dehydrogenation on Au-Based Catalysts at near-Ambient Temperatures. Angew. Chem. Int. Ed. 2009, 48, 4800–4803. [Google Scholar] [CrossRef]
- Zacharska, M.; Chuvilin, A.L.; Kriventsov, V.V.; Beloshapkin, S.; Estrada, M.; Simakov, A.; Bulushev, D.A. Support Effect for Nanosized Au Catalysts in Hydrogen Production from Formic Acid Decomposition. Catal. Sci. Technol. 2016, 6, 6853–6860. [Google Scholar] [CrossRef]
- Gazsi, A.; Bansagi, T.; Solymosi, F. Decomposition and Reforming of Formic Acid on Supported Au Catalysts: Production of CO-Free H2. J. Phys. Chem. C 2011, 115, 15459–15466. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Li, S.; Carrasquillo-Flores, R.; Alba-Rubio, A.C.; Dumesic, J.A.; Mavrikakis, M. Formic Acid Decomposition on Au Catalysts: DFT, Microkinetic Modeling, and Reaction Kinetics Experiments. AIChE J. 2014, 60, 1303–1319. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Guo, Y.; Beloshapkin, S.; Simakov, A. CO-Free Hydrogen Production from Decomposition of Formic Acid over Au/Al2O3 Catalysts Doped with Potassium Ions. Catal. Commun. 2017, 92, 86–89. [Google Scholar] [CrossRef]
- Jia, L.; Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. Hydrogen Production from Formic Acid Vapour over a Pd/C Catalyst Promoted by Potassium Salts: Evidence for Participation of Buffer-Like Solution in the Pores of the Catalyst. Appl. Catal. B Environ. 2014, 160, 35–43. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. Hydrogen from Formic Acid Decomposition over Pd and Au Catalysts. Catal. Today 2010, 154, 7–12. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/Porous Carbon Composites for Heterogeneous Catalysis: Old Catalysts with Improved Performance Promoted by N-Doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-Doped Carbon Materials as a Promising Platform toward the Efficient Catalysis for Hydrogen Generation. Appl. Catal. A Gen. 2019, 571, 25–41. [Google Scholar] [CrossRef]
- Kudashov, A.G.; Okotrub, A.V.; Yudanov, N.F.; Romanenko, A.I.; Bulusheva, L.G.; Abrosimov, O.G.; Chuvilin, A.L.; Pazhetov, E.M.; Boronin, A.I. Gas-Phase Synthesis of Nitrogen-Containing Carbon Nanotubes and Their Electronic Properties. Phys. Solid State 2002, 44, 652–655. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Ismagilov, Z.R. Nitrogen-Doped Carbon Nanomaterials: To the Mechanism of Growth, Electrical Conductivity and Application in Catalysis. Catal. Today 2015, 249, 12–22. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Zhang, T.; Di, X.; Ni, J.; Li, X. Enhancement of Au/AC Acetylene Hydrochlorination Catalyst Activity and Stability Via Nitrogen-Modified Activated Carbon Support. Chem. Eng. J. 2015, 262, 1152–1160. [Google Scholar] [CrossRef]
- Gil, S.; Lucas, P.J.; Nieto-Márquez, A.; Sánchez-Silva, L.; Giroir-Fendler, A.; Romero, A.; Valverde, J.L. Synthesis and Characterization of Nitrogen-Doped Carbon Nanospheres Decorated with Au Nanoparticles for the Liquid-Phase Oxidation of Glycerol. Ind. Eng. Chem. Res. 2014, 53, 16696–16706. [Google Scholar] [CrossRef]
- Dai, B.; Li, X.; Zhang, J.; Yu, F.; Zhu, M. Application of Mesoporous Carbon Nitride as a Support for an Au Catalyst for Acetylene Hydrochlorination. Chem. Eng. Sci. 2015, 135, 472–478. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Shlyakhova, E.V.; Chuvilin, A.L.; Guo, Y.; Beloshapkin, S.; Okotrub, A.V.; Bulusheva, L.G. Single Isolated Pd2+ Cations Supported on N-Doped Carbon as Active Sites for Hydrogen Production from Formic Acid Decomposition. ACS Catal. 2016, 6, 681–691. [Google Scholar] [CrossRef]
- Zacharska, M.; Bulusheva, L.G.; Lisitsyn, A.S.; Beloshapkin, S.; Guo, Y.; Chuvilin, A.L.; Shlyakhova, E.V.; Podyacheva, O.Y.; Leahy, J.J.; Okotrub, A.V.; et al. Factors Influencing the Performance of Pd/C Catalysts in the Green Production of Hydrogen from Formic Acid. ChemSusChem 2017, 10, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Podyacheva, O.Y.; Bulushev, D.A.; Suboch, A.N.; Svintsitskiy, D.A.; Lisitsyn, A.S.; Modin, E.; Chuvilin, A.; Gerasimov, E.Y.; Sobolev, V.I.; Parmon, V.N. Highly Stable Single-Atom Catalyst with Ionic Pd Active Sites Supported on N-Doped Carbon Nanotubes for Formic Acid Decomposition. ChemSusChem 2018, 11, 3724–3727. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Lisitsyn, A.S.; Podyacheva, O.Y.; Hage, F.S.; Ramasse, Q.M.; Bangert, U.; Bulusheva, L.G. Single Atoms of Pt-Group Metals Stabilized by N-Doped Carbon Nanofibers for Efficient Hydrogen Production from Formic Acid. ACS Catal. 2016, 6, 3442–3451. [Google Scholar] [CrossRef] [Green Version]
- Zacharska, M.; Podyacheva, O.Y.; Kibis, L.S.; Boronin, A.I.; Senkovskiy, B.V.; Gerasimov, E.Y.; Taran, O.P.; Ayusheev, A.B.; Parmon, V.N.; Leahy, J.J.; et al. Ruthenium Clusters on Carbon Nanofibers for Formic Acid Decomposition: Effect of Doping the Support with Nitrogen. ChemCatChem 2015, 7, 2910–2917. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Chuvilin, A.L.; Sobolev, V.I.; Stolyarova, S.G.; Shubin, Y.V.; Asanov, I.P.; Ishchenko, A.V.; Magnani, G.; Ricco, M.; Okotrub, A.V.; et al. Copper on Carbon Materials: Stabilization by Nitrogen Doping. J. Mater. Chem. A 2017, 5, 10574–10583. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Malykhin, S.E.; Svintsitskiy, D.A.; Podyacheva, O.Y.; Lisitsyn, A.S.; Richards, R.M. Nature of Active Palladium Sites on Nitrogen Doped Carbon Nanofibers in Selective Hydrogenation of Acetylene. Diam. Relat. Mater. 2018, 89, 67–73. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Lin, J.D.; Liu, Y.M.; He, H.Y.; Huang, F.Q.; Cao, Y. Dehydrogenation of Formic Acid at Room Temperature: Boosting Palladium Nanoparticle Efficiency by Coupling with Pyridinic-Nitrogen-Doped Carbon. Angew. Chem. Int. Ed. 2016, 55, 11849–11853. [Google Scholar] [CrossRef]
- Li, Z.P.; Yang, X.C.; Tsumori, N.; Liu, Z.; Himeda, Y.; Autrey, T.; Xu, Q. Tandem Nitrogen Functionalization of Porous Carbon: Toward Immobilizing Highly Active Palladium Nanoclusters for Dehydrogenation of Formic Acid. ACS Catal. 2017, 7, 2720–2724. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Chuvilin, A.L.; Sobolev, V.I.; Pirutko, L.V.; Fedoseeva, Y.V.; Lobiak, E.V.; Modin, E.; Okotrub, A.V.; Bulusheva, L.G. Single Au Atoms on the Surface of N-Free and N-Doped Carbon: Interaction with Formic Acid and Methanol Molecules. Top. Catal. 2019, in press. [Google Scholar]
- Peters, S.; Peredkov, S.; Neeb, M.; Eberhardt, W.; Al-Hada, M. Size-Dependent XPS Spectra of Small Supported Au-Clusters. Surf. Sci. 2013, 608, 129–134. [Google Scholar] [CrossRef]
- Liu, X.; Conte, M.; Elias, D.; Lu, L.; Morgan, D.J.; Freakley, S.J.; Johnston, P.; Kiely, C.J.; Hutchings, G.J. Investigation of the Active Species in the Carbon-Supported Gold Catalyst for Acetylene Hydrochlorination. Catal. Sci. Technol. 2016, 6, 5144–5153. [Google Scholar] [CrossRef]
- Malta, G.; Kondrat, S.A.; Freakley, S.J.; Davies, C.J.; Lu, L.; Dawson, S.; Thetford, A.; Gibson, E.K.; Morgan, D.J.; Jones, W.; et al. Identification of Single-Site Gold Catalysis in Acetylene Hydrochlorination. Science 2017, 355, 1399–1403. [Google Scholar] [CrossRef]
- Yi, N.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Hydrogen Production by Dehydrogenation of Formic Acid on Atomically Dispersed Gold on Ceria. ChemSusChem 2013, 6, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Primo, A.; Concepcion, P.; Alvaro, M.; Garcia, H. Doped Graphene as a Metal-Free Carbocatalyst for the Selective Aerobic Oxidation of Benzylic Hydrocarbons, Cyclooctane and Styrene. Chem. A Eur. J. 2013, 19, 7547–7554. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, G.; Zhong, J.; Shi, Z.; Zhu, Y.; Su, D.S.; Wang, J.; Bao, X.; Ma, D. Nitrogen-Doped sp2-Hybridized Carbon as a Superior Catalyst for Selective Oxidation. Angew. Chem. Int. Ed. 2013, 52, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Khavryuchenko, O.; Villa, A.; Su, D. Metal-Free Oxidation of Glycerol over Nitrogen-Containing Carbon Nanotubes. ChemSusChem 2017, 10, 3030–3034. [Google Scholar] [CrossRef]
- Ciftci, A.; Ligthart, D.A.J.M.; Pastorino, P.; Hensen, E.J.M. Nanostructured Ceria Supported Pt and Au Catalysts for the Reactions of Ethanol and Formic Acid. Appl. Catal. B Environ. 2013, 130–131, 325–335. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Du, X.L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Efficient Subnanometric Gold-Catalyzed Hydrogen Generation Via Formic Acid Decomposition under Ambient Conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Murugesan, T. Effect of Alkyl Chain Length on the Thermophysical Properties of Pyridinium Carboxylates. Chin. J. Chem. Eng. 2017, 25, 1266–1272. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Raubenheimer, H.G.; Dobrzanska, L. The Gold-Hydrogen Bond, Au-H, and the Hydrogen Bond to Gold, Au Center Dot Center Dot Center Dot H-X. Chem. Soc. Rev. 2014, 43, 345–380. [Google Scholar] [CrossRef]
- Shlyakhova, E.V.; Bulusheva, L.G.; Kanygin, M.A.; Plyusnin, P.E.; Kovalenko, K.A.; Senkovskiy, B.V.; Okotrub, A.V. Synthesis of Nitrogen-Containing Porous Carbon Using Calcium Oxide Nanoparticles. Phys. Status Solidi B 2014, 251, 2607–2612. [Google Scholar] [CrossRef]
- Ivanova, S.; Pitchon, V.; Zimmermann, Y.; Petit, C. Preparation of Alumina Supported Gold Catalysts: Influence of Washing Procedures, Mechanism of Particles Size Growth. Appl. Catal. A Gen. 2006, 298, 57–64. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Goerigk, L.; Grimme, S. A Thorough Benchmark of Density Functional Methods for General Main Group Thermochemistry, Kinetics, and Noncovalent Interactions. Phys. Chem. Chem. Phys. 2011, 11, 6670–6688. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]








| BET Surface Area of Support, m2 g−1 | Au Bulk Content, wt% | Au Surface Content, 3 wt% (XPS) | Au f7/2 (FWHM), 3 eV (XPS) | Mean Particle Size, 3 nm (TEM) | Reaction Rate at 448 K, 4 s−1 | Ea, kJ mol−1 | Selectivity to H2 at 448 K, % | |
|---|---|---|---|---|---|---|---|---|
| Au/C | 873 | 1.9 1 | 1.8 | 84.1 (0.9) | 10 ± 6 | 0.019 | 58 | 99.5 |
| Au/N-C | 674 | 0.7 1 | 0.8 | 83.9 (1.48) | 2.2 ± 0.9 | 0.045 | 53 | 96.6 |
| Au/Al2O3 | 200 | 1.8 2 | 1.5 | 83.9 (2.13) | 2.2 ± 1.0 | 0.017 | 48 | 98.0 |
| Au/SiO2 | 480 | 2.1 2 | 0.4 | 84.1 (2.22) | 1.6 ± 0.8 | 0.032 | 63 | 83.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulushev, D.A.; Sobolev, V.I.; Pirutko, L.V.; Starostina, A.V.; Asanov, I.P.; Modin, E.; Chuvilin, A.L.; Gupta, N.; Okotrub, A.V.; Bulusheva, L.G. Hydrogen Production from Formic Acid over Au Catalysts Supported on Carbon: Comparison with Au Catalysts Supported on SiO2 and Al2O3. Catalysts 2019, 9, 376. https://doi.org/10.3390/catal9040376
Bulushev DA, Sobolev VI, Pirutko LV, Starostina AV, Asanov IP, Modin E, Chuvilin AL, Gupta N, Okotrub AV, Bulusheva LG. Hydrogen Production from Formic Acid over Au Catalysts Supported on Carbon: Comparison with Au Catalysts Supported on SiO2 and Al2O3. Catalysts. 2019; 9(4):376. https://doi.org/10.3390/catal9040376
Chicago/Turabian StyleBulushev, Dmitri A., Vladimir I. Sobolev, Larisa V. Pirutko, Anna V. Starostina, Igor P. Asanov, Evgenii Modin, Andrey L. Chuvilin, Neeraj Gupta, Alexander V. Okotrub, and Lyubov G. Bulusheva. 2019. "Hydrogen Production from Formic Acid over Au Catalysts Supported on Carbon: Comparison with Au Catalysts Supported on SiO2 and Al2O3" Catalysts 9, no. 4: 376. https://doi.org/10.3390/catal9040376
APA StyleBulushev, D. A., Sobolev, V. I., Pirutko, L. V., Starostina, A. V., Asanov, I. P., Modin, E., Chuvilin, A. L., Gupta, N., Okotrub, A. V., & Bulusheva, L. G. (2019). Hydrogen Production from Formic Acid over Au Catalysts Supported on Carbon: Comparison with Au Catalysts Supported on SiO2 and Al2O3. Catalysts, 9(4), 376. https://doi.org/10.3390/catal9040376