Highly Loaded Mesoporous Ni–La2O3 Catalyst Prepared by Colloidal Solution Combustion Method for CO2 Methanation
Abstract
:1. Introduction
2. Results and Discussion
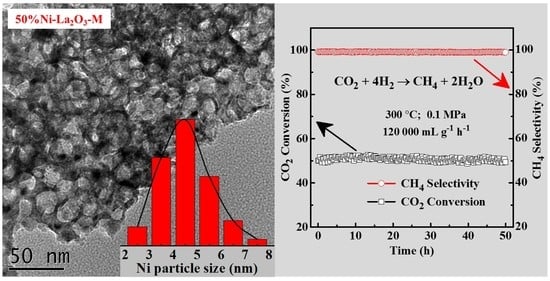
2.1. Characterization of Catalysts
2.2. Catalytic Performance
3. Materials and Methods
3.1. Synthesis of Catalysts
3.2. Characterization of Catalysts
3.3. Catalytic Performance Test for CO2 Methanation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Tan, M.; Zhang, P.; Fang, Y.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Rationally Designing Bifunctional Catalysts as an Efficient Strategy To Boost CO2 Hydrogenation Producing Value-Added Aromatics. ACS Catal. 2019, 9, 895–901. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Sharma, S.; Hu, Z.; Zhang, P.; McFarland, E.W.; Metiu, H. CO2 methanation on Ru-doped ceria. J. Catal. 2011, 278, 297–309. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Park, J.-N. Bifunctional Mechanism of CO2 Methanation on Pd-MgO/SiO2 Catalyst: Independent Roles of MgO and Pd on CO2 Methanation. J. Phys. Chem. C 2010, 114, 7128–7131. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Usman, M.; Suzuki, K.; Zhang, S. A highly active and stable Co4N/γ-Al2O3 catalyst for CO and CO2 methanation to produce synthetic natural gas (SNG). Chem. Eng. J. 2015, 262, 1090–1098. [Google Scholar] [CrossRef]
- Schubert, M.; Pokhrel, S.; Thomé, A.; Zielasek, V.; Gesing, T.M.; Roessner, F.; Mädler, L.; Bäumer, M. Highly active Co–Al2O3-based catalysts for CO2 methanation with very low platinum promotion prepared by double flame spray pyrolysis. Catal. Sci. Technol. 2016, 6, 7449–7460. [Google Scholar] [CrossRef]
- Liu, H.; Xu, S.; Zhou, G.; Huang, G.; Huang, S.; Xiong, K. CO2 hydrogenation to methane over Co/KIT-6 catalyst: Effect of reduction temperature. Chem. Eng. J. 2018, 351, 65–73. [Google Scholar] [CrossRef]
- Bian, Z.; Chan, Y.M.; Yu, Y.; Kawi, S. Morphology dependence of catalytic properties of Ni/CeO2 for CO2 methanation: A kinetic and mechanism study. Catal. Today 2018. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, Y. One-pot synthesis of NiO/SBA-15 monolith catalyst with a three-dimensional framework for CO2 methanation. Int. J. Hydrog. Energy 2017, 42, 12295–12300. [Google Scholar] [CrossRef]
- Chen, C.-S.; Budi, C.S.; Wu, H.-C.; Saikia, D.; Kao, H.-M. Size-Tunable Ni Nanoparticles Supported on Surface-Modified, Cage-Type Mesoporous Silica as Highly Active Catalysts for CO2 Hydrogenation. ACS Catal. 2017, 7, 8367–8381. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Micro- and mesoporous supports for CO2 methanation catalysts: A comparison between SBA-15, MCM-41 and USY zeolite. Chem. Eng. Sci. 2018, 175, 72–83. [Google Scholar] [CrossRef]
- Sun, X.; Suarez, A.I.O.; Meijerink, M.; van Deelen, T.; Ould-Chikh, S.; Zečević, J.; de Jong, K.P.; Kapteijn, F.; Gascon, J. Manufacture of highly loaded silica-supported cobalt Fischer–Tropsch catalysts from a metal organic framework. Nat. Commun. 2017, 8, 1680. [Google Scholar] [CrossRef]
- Garbarino, G.; Wang, C.; Cavattoni, T.; Finocchio, E.; Riani, P.; Flytzani-Stephanopoulos, M.; Busca, G. A study of Ni/La-Al2O3 catalysts: A competitive system for CO2 methanation. Appl. Catal. B 2019, 248, 286–297. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Zhao, J.; Chou, L. Methanation of Carbon Dioxide over a Highly Dispersed Ni/La2O3 Catalyst. Chin. J. Catal. 2010, 31, 21–23. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.-J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Chan, K.-Y.; Li, C.-Y.V. Colloidal Solution Combustion Synthesis: Toward Mass Production of a Crystalline Uniform Mesoporous CeO2 Catalyst with Tunable Porosity. Chem. Mater. 2016, 28, 2768–2775. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H. Mesoporous Co-CeO2 catalyst prepared by colloidal solution combustion method for reverse water-gas shift reaction. Catal. Today 2018, 316, 155–161. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Pereñíguez, R.; González-DelaCruz, V.M.; Holgado, J.P.; Caballero, A. Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts. Appl. Catal. B 2010, 93, 346–353. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y. NiO nanosheets grown on graphene nanosheets as superior anode materials for Li-ion batteries. Nanoscale 2011, 3, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, H.; Gong, D.; Ma, Z.; Liu, Y. Loading Ni/La2O3 on SiO2 for CO methanation from syngas. Catal. Today 2017, 297, 298–307. [Google Scholar] [CrossRef]
- Xue, Y.; Yan, C.; Zhao, X.; Huang, S.; Guo, C. Ni/La2O3-ZrO2 catalyst for hydrogen production from steam reforming of acetic acid as a model compound of bio-oil. Korean J. Chem. Eng. 2017, 34, 305–313. [Google Scholar] [CrossRef]
- Nuvula, S.; Sagar, T.V.; Valluri, D.K.; Sai Prasad, P.S. Selective substitution of Ni by Ti in LaNiO3 perovskites: A parameter governing the oxy-carbon dioxide reforming of methane. Int. J. Hydrog. Energy 2018, 43, 4136–4142. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Ye, H.; Hu, R.; Yang, S.; Tang, G.; Li, K.; Yang, Y. Vacuum Thermal Treated Ni-CeO2/SBA-15 Catalyst for CO2 Methanation. Nanomaterials 2018, 8, 759. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, S.; Gong, D.; Kang, N.; Fang, K.-G.; Liu, Y. Nano composite composed of MoOx-La2O3-Ni on SiO2 for storing hydrogen into CH4 via CO2 methanation. Int. J. Hydrog. Energy 2019, 44, 1597–1609. [Google Scholar] [CrossRef]







| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|
| Fresh 50% Ni–La2O3 | 7.2 | 0.13 | 71 |
| Fresh 50% Ni–La2O3–M | 70.4 | 0.32 | 18 |
| Reduced 50% Ni–La2O3–M a | 79.2 | 0.37 | 19 |
| Used 50% Ni–La2O3–M b | 55.5 | 0.45 | 16 |
| Catalysts | Crystal Size of NiO (nm) | Ni Crystal Size (nm) a | |
|---|---|---|---|
| By XRD a | By TEM b | ||
| Fresh 50% Ni–La2O3–M | 3.0 | 3.2 | / |
| Reduced 50% Ni–La2O3–M | / | / | 4.3 |
| Used 50% Ni–La2O3–M | / | / | 4.5 |
| Fresh 50% Ni–La2O3 | 7.0 | 6.8 | / |
| Reduced 50% Ni–La2O3 | / | / | 7.1 |
| Catalysts | Ni Dispersion (%) a | TOF (× 10−3 s−1) b |
|---|---|---|
| 50% Ni–La2O3 | 6.93 | 4.0 |
| 50% Ni–La2O3–M | 4.01 | 57.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, G.; Gong, D.; Liu, H.; Wang, L. Highly Loaded Mesoporous Ni–La2O3 Catalyst Prepared by Colloidal Solution Combustion Method for CO2 Methanation. Catalysts 2019, 9, 442. https://doi.org/10.3390/catal9050442
Tang G, Gong D, Liu H, Wang L. Highly Loaded Mesoporous Ni–La2O3 Catalyst Prepared by Colloidal Solution Combustion Method for CO2 Methanation. Catalysts. 2019; 9(5):442. https://doi.org/10.3390/catal9050442
Chicago/Turabian StyleTang, Guoli, Dandan Gong, Hui Liu, and Luhui Wang. 2019. "Highly Loaded Mesoporous Ni–La2O3 Catalyst Prepared by Colloidal Solution Combustion Method for CO2 Methanation" Catalysts 9, no. 5: 442. https://doi.org/10.3390/catal9050442
APA StyleTang, G., Gong, D., Liu, H., & Wang, L. (2019). Highly Loaded Mesoporous Ni–La2O3 Catalyst Prepared by Colloidal Solution Combustion Method for CO2 Methanation. Catalysts, 9(5), 442. https://doi.org/10.3390/catal9050442