Carbazolyl-Substituted [OSSO]-Type Zirconium(IV) Complex as a Precatalyst for the Oligomerization and Polymerization of α-Olefins
Abstract
:1. Introduction
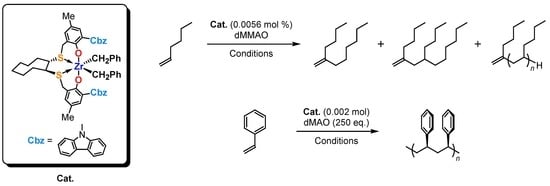
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of Dibenzyl Zirconium(IV) Complex 4
3.3. General Procedure of 1-Hexene Oligomerization
3.4. General Procedure for Styrene Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Resconi, L.; Chadwick, J.C.; Cavallo, L. Olefin polymerizations with group IV metal catalysts. In Comprehensive Organometallic Chemistry, 3rd ed.; Mingos, D.M.P., Crabtree, R.H., Eds.; Elsevier: Oxford, UK, 2016; Volume 4, pp. 1005–1166. [Google Scholar]
- Fujita, T.; Makio, H. Polymerization of alkenes. In Comprehensive Organometallic Chemistry, 3rd ed.; Mingos, D.M.P., Crabtree, R.H., Eds.; Elsevier: Oxford, UK, 2016; Volume 11, pp. 691–734. [Google Scholar]
- Baugh, L.S.; Canich, J.-A.M. Stereoselective Polymerization with Single-Site Catalysts; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Osakada, K. Organometallics Reactions and Polymerization; Springer: Heidelberg, Germany, 2014. [Google Scholar]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Suzuki, Y.; Terao, H.; Fujita, T. Recent advances in phenoxy-based catalysts for olefin polymerization. Bull. Chem. Soc. Jpn. 2003, 76, 1493–1517. [Google Scholar] [CrossRef]
- Givson, V.C.; Spitsmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar]
- Givson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar]
- Nakata, N.; Toda, T.; Ishii, A. Recent advances in the chemistry of Group 4 metal complexes incorporating [OSSO]-type bis(phenolato) ligands as post-metallocene catalysts. Polym. Chem. 2011, 2, 1597–1610. [Google Scholar] [CrossRef]
- Capacchione, C.; Proto, A.; Ebeling, H.; Mülhaupt, R.; Möller, K.; Spaniol, T.P.; Okuda, J. Ancillary ligand effect on single-site styrene polymerization: Isospecificity of group 4 metal bis(phenolate) catalysts. J. Am. Chem. Soc. 2003, 125, 4964–4965. [Google Scholar] [CrossRef] [PubMed]
- Proto, A.; Capacchione, C.; Venditto, V.; Okuda, J. Synthesis of isotactic poly-1,2-(4-methyl-1,3-pentadiene) by a homogeneous titanium catalyst. Macromolecules 2003, 36, 9249–9251. [Google Scholar] [CrossRef]
- Capacchione, C.; Proto, A.; Okuda, J. Synthesis of branched polyethylene by ethylene homopolymerization using titanium catalysts that contain a bridged bis(phenolate) ligand. J. Polym. Sci. Part A: Polym. Chem. 2004, 42, 2815–2822. [Google Scholar] [CrossRef]
- Beckerle, K.; Capacchione, C.; Ebeling, H.; Manivannan, R.; Mülhaupt, R.; Proto, A.; Spaniol, T.P.; Okuda, J. Stereospecific post-metallocene polymerization catalysts: The example of isospecific styrene polymerization. J. Organomet. Chem. 2004, 689, 4636–4641. [Google Scholar] [CrossRef]
- Capacchione, C.; De Carlo, F.; Zannoni, C.; Okuda, J.; Proto, A. Propylene−styrene multiblock copolymers: evidence for monomer enchainment via opposite insertion regiochemistry by a single-site catalyst. Macromolecules 2004, 37, 8918–8922. [Google Scholar] [CrossRef]
- Capacchione, C.; Manivannan, R.; Barone, M.; Beckerle, K.; Centore, R.; Oliva, L.; Proto, A.; Tuzi, A.; Spaniol, T.P.; Okuda, J. Isospecific styrene polymerization by chiral titanium complexes that contain a tetradentate [OSSO]-type bis(phenolato) ligand. Organometallics 2005, 24, 2971–2982. [Google Scholar] [CrossRef]
- Beckerle, K.; Manivannan, R.; Spaniol, T.P.; Okuda, J. Living isospecific styrene polymerization by chiral benzyl titanium complexes that contain a tetradentate [OSSO]-type bis(phenolato) ligand. Organometallics 2006, 25, 3019–3026. [Google Scholar] [CrossRef]
- Lian, B.; Beckerle, K.; Spaniol, T.P.; Okuda, J. Stereospecific styrene enchainment at a titanium site within a helical ligand framework: Evidence for the formation of homochiral polystyrene. Angew. Chem. Int. Ed. 2006, 46, 8507–8510. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Yeori, A.; Goldberg, I.; Kol, M. Group 4 complexes of a new [OSSO]-type dianionic ligand. coordination chemistry and preliminary polymerization catalysis studies. Inorg. Chem. 2007, 46, 8114–8116. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Cuomo, C.; Capacchione, C.; Zannoni, C.; Grassi, A.; Proto, A. Stereoselective polymerization of conjugated dienes and styrene–butadiene copolymerization promoted by octahedral titanium catalyst. Macromolecules 2007, 40, 5638–5643. [Google Scholar]
- Capacchione, C.; Avagliano, A.; Proto, A. Ethylene–butadiene copolymerization promoted by titanium complex containing a tetradentate [OSSO]-type bis(phenolato) ligand. Macromolecules 2008, 41, 4573–4575. [Google Scholar] [CrossRef]
- Gall, B.; Pelascini, F.; Ebeling, H.; Beckerle, K.; Okuda, J.; Mülhaupt, R. Molecular weight and end group control of isotactic polystyrene using olefins and nonconjugated diolefins as chain transfer agents. Macromolecules 2008, 41, 1627–1633. [Google Scholar] [CrossRef]
- Proto, A.; Avagliano, A.; Saviello, D.; Capacchione, C. Copolymerization of ethylene with 4-methyl-1,3-pentadiene promoted by titanium complexes containing a tetradentate [OSSO]-type bis(phenolato) ligand. Macromolecules 2009, 42, 6981–6985. [Google Scholar] [CrossRef]
- Sergeeva, E.; Kopilov, J.; Goldberg, I.; Kol, M. Dithiodiolate ligands: Group 4 complexes and application in lactide polymerization. Inorg. Chem. 2010, 49, 3977–3979. [Google Scholar] [CrossRef]
- Proto, A.; Avagliano, A.; Saviello, D.; Ricciardi, R.; Capacchione, C. Living, isoselective polymerization of styrene and formation of stereoregular block copolymers via sequential monomer addition. Macromolecules 2010, 43, 5919–5921. [Google Scholar] [CrossRef]
- Capacchione, C.; Saviello, D.; Avagliano, A.; Proto, A. Copolymerization of ethylene with isoprene promoted by titanium complexes containing a tetradentate [OSSO]-type bis(phenolato) ligand. J. Polym. Sci. Part A: Polym. Chem. 2010, 48, 4200–4206. [Google Scholar] [CrossRef]
- Galdi, N.; Santoro, O.; Oliva, L.; Proto, A.; Capacchione, C. Asymmetric hydrodimerization of styrene by a chiral zirconium complex containing a tetradentate [OSSO]-type bis(phenolato) ligand. Catal. Commun. 2011, 12, 1113–1117. [Google Scholar] [CrossRef]
- Konkol, M.; Nabika, M.; Kohno, T.; Hino, T.; Miyatake, T. Synthesis, structure and α-olefin polymerization activity of group 4 metal complexes with [OSSO]-type bis(phenolate) ligands. J. Organomet. Chem. 2011, 696, 1792–1802. [Google Scholar] [CrossRef]
- Ishii, A.; Asajima, K.; Toda, T.; Nakata, N. Synthesis of titanium(IV) and zirconium(IV) complexes with an [OSSO]-type bis(phenolate) ligand bearing a trans-cyclohexane-1,2-diyl ring and 1-hexene polymerization. Organometallics 2011, 30, 2947–2956. [Google Scholar] [CrossRef]
- Cohen, A.; Goldberg, I.; Venditto, V.; Kol, M. Oscillating non-metallocenes - from stereoblock-isotactic polypropylene to isotactic polypropylene via zirconium and hafnium dithiodiphenolate catalysts. Eur. J. Inorg. Chem. 2011, 5219–5223. [Google Scholar] [CrossRef]
- Capacchione, C.; Saviello, D.; Ricciardi, R.; Proto, A. Living, isoselective polymerization of 4-methyl-1,3-pentadiene and styrenic monomers and synthesis of highly stereoregular block copolymers via sequential monomer addition. Macromolecules 2011, 44, 7940–7947. [Google Scholar] [CrossRef]
- Hu, P.; Wang, J.Q.; Wang, F.; Jin, G.X. Preparation, structure, and ethylene (co)polymerization behavior of group IV metal complexes with an [OSSO]-carborane ligand. Chem. Eur. J. 2011, 17, 8576–8583. [Google Scholar] [CrossRef] [PubMed]
- Costabile, C.; Capacchione, C.; Saviello, D.; Proto, A. Mechanistic studies on conjugated diene polymerizations promoted by a titanium complex containing a tetradentate [OSSO]-type bis(phenolato) ligand. Macromolecules 2012, 45, 6363–6370. [Google Scholar] [CrossRef]
- Buonerba, A.; Fienga, M.; Milione, S.; Cuomo, C.; Grassi, A.; Proto, A.; Capacchione, C. Binary copolymerization of p-methylstyrene with butadiene and isoprene catalyzed by titanium compounds showing different stereoselectivity. Macromolecules 2013, 46, 8449–8457. [Google Scholar] [CrossRef]
- Hohberger, C.; Spaniol, T.P.; Okuda, J. Living polymerization by bis(phenolate) zirconium catalysts: Synthesis of isotactic polystyrene-block-polybutadiene copolymers. Macromol. Chem. Phys. 2014, 215, 2001–2006. [Google Scholar] [CrossRef]
- Lapenta, R.; Buonerba, A.; De Nisi, A.; Monari, M.; Grassi, A.; Milione, S.; Capacchione, C. Stereorigid OSSO-type group 4 metal complexes in the ring-opening polymerization of rac-lactide. Inorg. Chem. 2017, 56, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Ikuma, K.; Nakata, N.; Nakamura, K.; Kuribayashi, H.; Takaoki, K. Zirconium and hafnium complexes with cycloheptane- or cyclononane-fused [OSSO]-type bis(phenolato) ligands: Synthesis, structure, and highly active 1-hexene polymerization and ring-size effects of fused cycloalkanes on the activity. Organometallics 2017, 36, 3954–3966. [Google Scholar] [CrossRef]
- Lapenta, R.; Buonerba, A.; Luciano, E.; Della Monica, F.; De Nisi, A.; Monari, M.; Grassi, A.; Capacchione, C.; Milione, C. Phenylene-bridged OSSO-type titanium complexes in the polymerization of ethylene and propylene. ACS Omega 2018, 3, 11608–11616. [Google Scholar] [CrossRef]
- Nakata, N.; Ishii, A. Precise polymerization of α-olefins using a mixed donor-type ligand containing oxygen and sulfur atoms. Koubunshi Ronbunshu 2015, 72, 285–294. [Google Scholar] [CrossRef]
- Ishii, A.; Toda, T.; Nakata, N.; Matsuo, T. Zirconium complex of an [OSSO]-type diphenolate ligand bearing trans-1,2-cyclooctanediylbis(thio) core: Synthesis, structure, and isospecific 1-hexene polymerization. J. Am. Chem. Soc. 2009, 131, 13566–13567. [Google Scholar] [CrossRef] [PubMed]
- Nakata, N.; Toda, T.; Ishii, A.; Matsuo, T. Titanium complexes supported by an [OSSO]-type bis(phenolato) ligand based on a trans-cyclooctanediyl platform: Synthesis, structures, and 1-hexene polymerization. Inorg. Chem. 2012, 51, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Nakata, N.; Ishii, A.; Matsuo, T. Synthesis, structure, and 1-hexene polymerization catalytic ability of group 5 metal complexes incorporating an [OSSO]-type ligand. ACS Catal. 2013, 3, 1764–1767. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled isospecific polymerization of α-olefins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis(phenolate) ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar] [CrossRef]
- Toda, T.; Nakata, N.; Matsuo, T.; Ishii, A. Extremely active α-olefin polymerization and copolymerization with ethylene catalyzed by a dMAO-activated zirconium(IV) dichloro complex having an [OSSO]-type ligand. RSC Adv. 2015, 8, 88826–88831. [Google Scholar] [CrossRef]
- Saito, Y.; Nakata, N.; Ishii, A. Highly isospecific polymerization of silyl-protected ω-alkenols with an [OSSO]-type bis(phenolato) dichloro zirconium(IV) precatalyst. Macromol. Rapid Commun. 2016, 37, 969–974. [Google Scholar] [CrossRef]
- Nakata, N.; Watanabe, T.; Toda, T.; Ishii, A. Enantio- and stereoselective cyclopolymerization of hexa-1,5-diene catalyzed by zirconium complexes possessing optically active bis(phenolato) ligands. Macromol. Rapid Commun. 2016, 37, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Nakata, N.; Toda, T.; Saito, Y.; Watanabe, T.; Ishii, A. Highly active and isospecific styrene polymerization catalyzed by zirconium complexes bearing aryl-substituted [OSSO]-type bis(phenolate) ligands. Polymers 2016, 31, 8. [Google Scholar] [CrossRef] [PubMed]
- Nakata, N.; Nakamura, K.; Ishii, A. Highly efficient and 1,2-regioselective method for the oligomerization of 1-hexene promoted by zirconium precatalysts with [OSSO]-type bis(phenolate) ligands. Organometallics 2018, 37, 2640–2644. [Google Scholar] [CrossRef]
- Takano, M.; Ito, K.; Ishii, A.; Nakata, N.; Kawauchi, F. JP 2013166735. Jpn. Kokai Tokkyo Koho 2013. [Google Scholar]
- Harvey, B.G.; Meylemans, H.A. 1-Hexene: A renewable C6 platform for full-performance jet and diesel fuels. Green Chem. 2014, 16, 770–776. [Google Scholar] [CrossRef]
- Harwood, H.J.; Chen, T.K.; Lin, F.T. 75-MHz 13C NMR Studies on Polystyrene and Epimerized Isotactic Polystyrenes. ACS Symp. Ser. 1984, 247, 197–222. [Google Scholar]
- Rodrigues, A.-S.; Kirillov, E.; Roisnel, T.; Razavi, A.; Vuillemin, B.; Carpentier, J.-F. Highly isospecific styrene polymerization catalyzed by single-component bridged bis(indenyl) allyl yttrium and neodymium complexes. Angew. Chem. Int. Ed. 2007, 46, 7240–7243. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Kawahara, T.; Shinto, Y.; Nakayama, Y.; Shiono, T. An alternative method for the preparation of trialkylaluminum-depleted modified methylaluminoxane (dMMAO). Macromolecules 2017, 50, 5989–5993. [Google Scholar] [CrossRef]
- Hasan, T.; Ioku, A.; Nishii, K.; Shiono, T.; Ikeda, T. Syndiospecific living polymerization of propene with [t-BuNSiMe2Flu]TiMe2 using MAO as cocatalyst. Macromolecules 2001, 34, 3142–3145. [Google Scholar] [CrossRef]
- Rong, Y.; Al-Harbi, A.; Parkin, G. Highly variable Zr-CH2-Ph bond angles in tetrabenzylzirconium: Analysis of benzyl ligand coordination modes. Organometallics 2012, 31, 8208–8217. [Google Scholar] [CrossRef]



| Oligomer Distribution c [%] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Run | Al/Zr | Temp. [°C] | Conv. [%] | TOF [h−1] | Vinylidene Selectivity b [%] | Dimer | Trimer | Others |
| 1 | 100 | 25 | 6.3 | 1130 | 98 | 39 | 34 | 27 |
| 2 | 150 | 25 | 7.6 | 1350 | 98 | 62 | 21 | 17 |
| 3 | 200 | 25 | 8.6 | 1540 | 98 | 56 | 25 | 18 |
| 4 | 250 | 25 | 9.5 | 1690 | 98 | 55 | 25 | 20 |
| 5 | 250 | 40 | 11.7 | 2080 | 98 | 53 | 26 | 21 |

| Run | Temp. [°C] | Yield [g] | Activity [g mmol−1 h−1] | Mw [g mol−1] | PDI b | [mm] c [%] |
|---|---|---|---|---|---|---|
| 6 | 0 | 0.085 | 85 | 223,000 | 20.3 | >99 |
| 7 | 25 | 0.414 | 414 | 508,000 | 4.2 | >99 |
| 8 | 40 | 1.00 | 1000 | 458,000 | 2.8 | >99 |
| 9 | 70 | 2.81 | 2810 | 179,000 | 2.1 | >99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, N.; Nakamura, K.; Nagaoka, S.; Ishii, A. Carbazolyl-Substituted [OSSO]-Type Zirconium(IV) Complex as a Precatalyst for the Oligomerization and Polymerization of α-Olefins. Catalysts 2019, 9, 528. https://doi.org/10.3390/catal9060528
Nakata N, Nakamura K, Nagaoka S, Ishii A. Carbazolyl-Substituted [OSSO]-Type Zirconium(IV) Complex as a Precatalyst for the Oligomerization and Polymerization of α-Olefins. Catalysts. 2019; 9(6):528. https://doi.org/10.3390/catal9060528
Chicago/Turabian StyleNakata, Norio, Kazuaki Nakamura, Shotaro Nagaoka, and Akihiko Ishii. 2019. "Carbazolyl-Substituted [OSSO]-Type Zirconium(IV) Complex as a Precatalyst for the Oligomerization and Polymerization of α-Olefins" Catalysts 9, no. 6: 528. https://doi.org/10.3390/catal9060528
APA StyleNakata, N., Nakamura, K., Nagaoka, S., & Ishii, A. (2019). Carbazolyl-Substituted [OSSO]-Type Zirconium(IV) Complex as a Precatalyst for the Oligomerization and Polymerization of α-Olefins. Catalysts, 9(6), 528. https://doi.org/10.3390/catal9060528