Catalyst/Feedstock Ratio Effect on FCC Using Different Catalysts Samples
Abstract
:1. Introduction
2. Results of the Catalyst Characterization
2.1. X-Ray Diffraction Analysis
2.2. NH3-TPD (Temperature Programmed Desorption)
2.3. Pyridine-FTIR
2.4. N2 Adsorption-Desorption Isotherms
3. Thermal Cracking Runs
4. Catalytic Cracking Runs
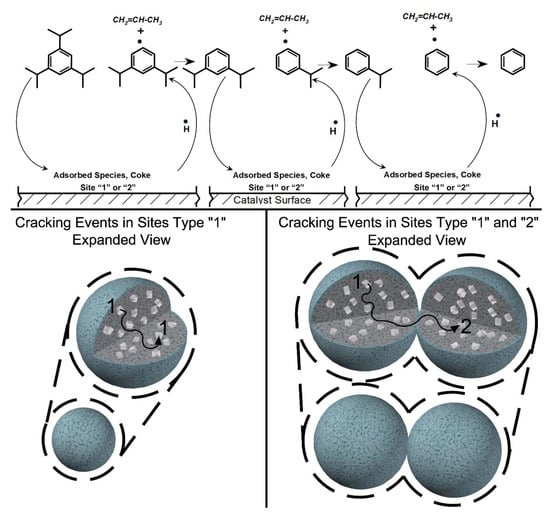
4.1. Effect of Catalyst-to-Oil Ratio (Catoil C/O Ratio) (g cat g feed −1)
4.2. Coke Selectivity
4.3. Hydrocarbon Product Selectivity
5. Materials and Methods
5.1. Feedstock and Catalysts
5.2. Catalyst Characterization
5.3. Chemical Species Analytical Methods
5.4. Catalytic Cracking Evaluation
6. Conclusions
- (a)
- It is shown that the 1,3,5-TIPB catalytic cracking displays common activity trends at increasing C/O ratios. This is shown using three Y-zeolite catalysts with different acidities and crystallinities.
- (b)
- It is proven that the 1,3,5-TIPB catalytic cracking, when using these three catalysts, displays maximum 1,3,5 TIPB conversions at C/O ratios of 2.5.
- (c)
- It is observed that the 1,3,5-TIPB cracking product selectivity shows the highest coke and the highest undesirable benzene selectivity at the maximum C/O of 5.
- (d)
- It is thus anticipated that catalyst density, which is a main parameter that determines the C/O ratio, plays a critical role in achieving the highest 1,3,5-TIPB conversions. This phenomenon is of significant importance for the operation of scaled FCC units.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| D | Pore diameter (A°) |
| Number of moles of “i” species (moles) in the gas phase | |
| Number of moles of coke collected (moles) | |
| T | Reaction temperature, K |
| t | Reaction time, s |
| Mass of catalyst (g) loaded in the riser (basket), where catalytic cracking occurs. | |
| Total mass of hydrocarbons injected (g) |
Abreviations
| BAS | Brönsted acid sites |
| BET | Brunauer–Emmett–Teller |
| CAT-A | Catalyst A |
| CAT-B | Catalyst B |
| CAT-C | Catalyst C |
| Catoil (C/O) | Catalyst-to-oil ratio (g/g) |
| CREC | Chemical Reaction Engineering Center |
| DRIFTS | Diffuse reflectance infrared Fourier transform spectroscopy |
| FCC | Fluidized catalytic cracking |
| FID | Flame ionization detector |
| FTIR | Fourier transform infrared spectroscopy |
| GC | Gas chromatography |
| LAS | Lewis acid sites |
| MSD | Mass spectrometer detector |
| MAT | Micro activity tests units |
| PSD | Pore size distribution |
| PV | Pore volume (cm3/g) |
| Selectivity-M | Moles of product “i” species/moles of 1,3,5-TIPB converted |
| Selectivity-W | Grams of coke/grams of TIPB converted. |
| SSA | Specific surface area (m2/g) |
| TCD | Thermal conductivity detector |
| 1,3,5-TIPB | 1,3,5-Tri-isopropyl benzene |
| TOC | Total organic coke (g-coke/g-cat.) |
| TPD | Temperature programmed desorption |
| VGO | Vacuum gas oil |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Hussain, A.I.; Palani, A.; Aitani, A.M.; Shamzhy, M.; Kub, M.; Al-khattaf, S.S. Catalytic cracking of vacuum gasoil over-SVR, ITH, and MFI zeolites as FCC catalyst additives. Fuel Process. Technol. 2017, 161, 23–32. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuyse, B.M. Fluid catalytic cracking: Recent development on the grand and old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Lu, C.; Wang, Z.; Gao, X. Quantitative correlations of cracking performance with physiochemical properties of FCC catalysts by a novel lump kinetic modelling method. Fuel 2015, 161, 113–119. [Google Scholar] [CrossRef]
- Corma, A.; Sauvanaud, L. FCC testing at bench scale: New units, new processes, new feeds. Catal. Today 2013, 218–219, 107–114. [Google Scholar] [CrossRef]
- Passamonti, F.; de la Puente, G.; Gilbert, W.; Morgado, E.; Sedran, U. Comparison between fixed fluidized bed (FFB) and batch fluidized bed reactors in the evaluation of FCC catalysts. Chem. Eng. J. 2012, 183, 433–447. [Google Scholar] [CrossRef]
- Marin, G.B. Rigorous Kinetic Models for Catalyst Deactivation by Coke Deposition: Application to Butene Dehydrogenation. J. Catal. 1986, 426, 416–426. [Google Scholar] [CrossRef]
- Cerqueira, H.S.; Caeiro, G.; Costa, L.; Ribeiro, F.R. Deactivation of FCC catalysts. J. Mol. Catal. Chem. 2008, 292, 1–13. [Google Scholar] [CrossRef]
- Bai, T.; Zhang, X.; Wang, F.; Qu, W.; Liu, X.; Duan, C. Coking behaviors and kinetics on HZSM-5/SAPO-34 catalysts for conversion of ethanol to propylene. J. Energy Chem. 2016, 25, 545–552. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kumar, V.; Srivastava, V.K. Modeling of Fluid Catalytic Cracking Riser Reactor: A Review. Int. J. Chem. React. Eng. 2010, 8, 1–40. [Google Scholar] [CrossRef]
- Han, I.; Riggs, J.B.; Chung, C. Modeling and optimization of a fluidized catalytic cracking process under full and partial combustion modes. Chem. Eng. Process. Process Intensif. 2004, 43, 1063–1084. [Google Scholar] [CrossRef]
- Al-Khattaf, S.; Saeed, M.R.; Aitani, A.; Klein, M.T. Catalytic cracking of light crude oil to Light Olefins and naphtha over E-Cat and MFI: Microactivity Test versus Advanced cracking evaluation and the effect of high reaction temperature. Energy Fuels 2018, 32, 6189–6199. [Google Scholar] [CrossRef]
- Wallensteln, D. Rational assessment of FCC catalyst performance by utilization of micro-activity testing. Appl. Catal. A Gen. 1998, 167, 141–155. [Google Scholar] [CrossRef]
- Shan, H.; Dong, H.; Zhang, J.; Niu, G. Experimental study of two-stage riser FCC reactions. Fuel 2001, 80, 1179–1185. [Google Scholar] [CrossRef]
- Gianetto, A.; Farag, H.I.; Blasetti, A.P.; de Lasa, H. Fluid Catalytic Cracking Catalyst for Reformulated Gasolines. Kinet. Modeling 1994, 33, 3053–3062. [Google Scholar]
- Delattre, C.; Forissier, M.; Pitault, I.; Schweich, D.; Bernard, J.R. Improvement of the microactivity test for kinetic and deactivation studies involved in catalytic cracking. Chem. Eng. Sci. 2001, 56, 1337–1345. [Google Scholar] [CrossRef]
- Fernandes, J.L.; Domingues, L.H.; Pinheiro, C.I.C.; Oliveira, N.M.C.; Ramôa, F. Influence of different catalyst deactivation models in a validated simulator of an industrial UOP FCC unit with high-efficiency regenerator. Fuel 2012, 97, 97–108. [Google Scholar] [CrossRef]
- Pinheiro, C.I.; Fernandes, J.L.; Domingues, L.; Chambel, A.J.; Graca, I.; Oliveira, N.M.; Cerqueira, H.S.; Ribeiro, F.R. Fluid Catalytic Cracking (FCC) Process Modeling, Simulation, and Control. Ind. Eng. Chem. Res. 2012, 51, 1–29. [Google Scholar] [CrossRef]
- Dupain, X.; Makkee, M.; Moulijn, J.A. Optimal conditions in fluid catalytic cracking: A mechanistic approach. Appl. Catal. A Gen. 2006, 297, 198–219. [Google Scholar] [CrossRef]
- Lappas, A.A.; Iatridis, D.K.; Papapetrou, M.C.; Kopalidou, E.P.; Vasalos, I.A. Feedstock and catalyst effects in fluid catalytic cracking—Comparative yields in bench scale and pilot plant reactors. Chem. Eng. J. 2015, 278, 140–149. [Google Scholar] [CrossRef]
- Jiménez-García, G.; Quintana-Solórzano, R.; Maya-Yescas, R. Improving accuracy in the estimation of kinetic frequency factors from laboratory data to model industrial catalytic cracking risers. Ind. Eng. Chem. Res. 2011, 50, 2736–2745. [Google Scholar] [CrossRef]
- de Lasa, H.I. Riser Simulator. U.S. Patent No. 5,102,628, 7 April 1992. [Google Scholar]
- Passamonti, F.J.; Puente, G.D.; Sedran, U. Comparison between MAT flow fixed bed and batch fluidized bed reactors in the evaluation of FCC catalysts. 2. Naphtha composition. Energy Fuels 2009, 23, 3510–3516. [Google Scholar] [CrossRef]
- Pruski, J.; Pekediz, A.; de Lasa, H. Catalytic cracking of hydrocarbons in a novel Riser Simulator: Lump Adsorption parameters under reaction conditions. Chem. Eng. Sci. 1996, 5, 1799–1806. [Google Scholar] [CrossRef]
- Kraemer, D.W. Modelling Catalytic Cracking in A Novel Riser Simulator (volumes I and Ii). Ph.D. Thesis, Western University, London, ON, Canada, 1991. [Google Scholar]
- Bartholomew, C.H.; Argyle, M.D. Advances in Catalyst Deactivation and Regeneration. Catalysts 2015, 5, 949–954. [Google Scholar] [CrossRef]
- Jarullah, A.T.; Awad, N.A.; Mujtaba, I.M. Optimal design and operation of an industrial fluidized catalytic cracking reactor. Fuel 2017, 206, 657–674. [Google Scholar] [CrossRef] [Green Version]
- Al-Sabawi, M.; Atias, J.A.; de Lasa, H. Kinetic modeling of catalytic cracking of gas oil feedstocks: Reaction and diffusion phenomena. Ind. Eng. Chem. Res. 2006, 45, 1583–1593. [Google Scholar] [CrossRef]
- Usman, A.; Bari, M.A.; Hussain, A.; Aitani, A.; Al-khattaf, S. Chemical engineering research and design catalytic cracking of crude oil to light olefins and naphtha: Experimental and kinetic modeling. Chem. Eng. Res. Des. 2017, 120, 121–137. [Google Scholar] [CrossRef]
- Yang, G.-F.; Wang, G.; Gao, J.S.; Xu, C.M. Coke formation and olefins conversion in FCC naphthaolefin reformulation at low reaction temperature. J. Fuel Chem. Technol. 2007, 35, 572–577. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, J.; Yang, C. Effects of temperature and catalyst to oil weight ratio on the catalytic conversion of heavy oil to propylene using ZSM-5 and USY catalysts. J. Nat. Gas Chem. 2007, 16, 92–99. [Google Scholar] [CrossRef]
- Alkhlel, A.; de Lasa, H. Catalytic Cracking of Hydrocarbons in a CREC Riser Simulator Using a Y—Zeolite-Based Catalyst: Assessing the Catalyst/Oil Ratio Effect. Ind. Eng. Chem. Res. 2018, 57, 13627–13638. [Google Scholar] [CrossRef]
- Alkhlel, A. Catalytic Conversion of Hydrocarbon 1,3,5-TIPB Over Y-Zeolite based Catalysts, Catalyst/oil ratio(C/O) Effect, and a Kinetic Model. Ph.D. Thesis. Disertatoion University of Western Ontario to be submitted August 2019.
- Jimeńez-García, G.; de Lasa, H.; Quintana-Soloŕzano, R.; Maya-Yescas, R. Catalyst activity decay due to pore blockage during catalytic cracking of hydrocarbons. Fuel 2013, 110, 89–98. [Google Scholar] [CrossRef]
- Atias, J.; Tonetto, G.M.; de Lasa, H. Modeling fluid catalytic cracking in a novel CREC Riser Simulator: Adsorption parameters under reaction conditions. Int. J. Chem. React. Eng. 2003, 1, 1–23. [Google Scholar] [CrossRef]
- Al-Khattaf, S.; de Lasa, H. The role of diffusion in alkyl-benzenes catalytic cracking. Appl. Catal. A Gen. 2002, 226, 139–153. [Google Scholar] [CrossRef]
- Mi, S.; Wei, T.; Sun, J.; Liu, P.; Li, X.; Zheng, Q.; Gong, K.; Liu, X. Catalytic function of boron to creating interconnected mesoporosity in microporous Y zeolites and its high performance in hydrocarbon cracking. J. Catal. 2017, 347, 116–126. [Google Scholar] [CrossRef]
- Guo, D.; Shen, B.; Qin, Y.; Sun, J.; Guo, Q.; Ren, S.; Gao, X.; Pang, X.; Wang, B.; Zhao, H. Microporous and Mesoporous Materials USY zeolites with tunable mesoporosity designed by controlling framework Fe content and their catalytic cracking properties. Microporous Mesoporous Mater. 2015, 211, 192–199. [Google Scholar] [CrossRef]
- Atias, J.A.; de Lasa, H. Adsorption, diffusion, and reaction phenomena on FCC catalysts in the CREC riser simulator. Ind. Eng. Chem. Res. 2004, 43, 4709–4720. [Google Scholar] [CrossRef]
- Bazyari, A.; Khodadadi, A.A.; Hosseinpour, N.; Mortazavi, Y. Effects of steaming-made changes in physicochemical properties of Y-zeolite on cracking of bulky 1,3,5-triisopropylbenzene and coke formation. Fuel Process. Technol. 2009, 90, 1226–1233. [Google Scholar] [CrossRef]
- White, M.G. Heterogeneous Catalysis; Prentice Hall: Upper Saddle River, NJ, USA, 1992. [Google Scholar]
- Tonetto, G.; Atias, J.; de Lasa, H. FCC catalysts with different zeolite crystallite sizes: Acidity, structural properties and reactivity. Appl. Catal. A Gen. 2004, 270, 9–25. [Google Scholar] [CrossRef]
- Arandes, J.M.; Abajo, I.; Fernández, I.; Azkoiti, M.J.; Bilbao, J. Effect of HZSM-5 Zeolite Addition to a Fluid Catalytic Cracking Catalyst. Study in a Laboratory Reactor Operating under Industrial Conditions. Ind. Eng. Chem. Res. 2000, 39, 1917–1924. [Google Scholar] [CrossRef]










| Sample | Particle SiO2/Al2O3(mol/mol) | Unit Cell Size (A°) | Crystallinity |
|---|---|---|---|
| CAT-A | 0.86 | 24.30 | 0.094 |
| CAT-B | 0.72 | 24.30 | 0.077 |
| CAT-C | 0.92 | 24.29 | 0.078 |
| CAT-A | CAT-B | CAT-C | |
|---|---|---|---|
| Sample | NH3 uptake (cm3 STP/g) | NH3 uptake (cm3 STP/g) | NH3 uptake (cm3 STP/g) |
| Free of Coke | 3.36 | 1.73 | 1.47 |
| Catoil = 1.25 | 2.48 | 1.24 | 1.14 |
| Catoil = 2.5 | 2.31 | 1.24 | 1.13 |
| Catoil = 3.75 | 2.24 | 1.04 | 0.94 |
| Catoil = 5 | 2.23 | 0.95 | 0.84 |
| Sample Catalyst | Brönsted/Lewis Acid Sites Ratio |
|---|---|
| CAT-A | 0.298 |
| CAT-B | 0.334 |
| CAT-C | 0.321 |
| CAT-A | CAT-B | CAT-C | |
|---|---|---|---|
| BET (SSA) | 99.6 | 118.5 | 102 |
| Pore Volume (PV) (cm3/g) | 0.158 | 0.196 | 0.140 |
| Mesopores Volume (cm3/g) | 0.112 | 0.142 | 0.0916 |
| Micropores Volume (cm3/g) | 0.0468 | 0.0540 | 0.0491 |
| CAT-B Catalyst Samples | ||||||
|---|---|---|---|---|---|---|
| Free Coke | C/O = 0.6 | C/O = 0.8 | C/O = 1.25 | C/O = 2.5 | C/O = 3.75 | |
| BET (Specific Surface Area) | 102 | 93.37 | 91.88 | 89.18 | 88.92 | 81.56 |
| Pore Volume (PV) cm3/g | 0.140 | 0.129 | 0.137 | 0.121 | 0.122 | 0.120 |
| Mesopores-Macropores Volume, cm3/g | 0.0916 | 0.0864 | 0.0936 | 0.0785 | 0.0795 | 0.0815 |
| Micropores Volume cm3/g | 0.0491 | 0.0435 | 0.0439 | 0.0430 | 0.0425 | 0.0390 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhlel, A.; de Lasa, H. Catalyst/Feedstock Ratio Effect on FCC Using Different Catalysts Samples. Catalysts 2019, 9, 542. https://doi.org/10.3390/catal9060542
Alkhlel A, de Lasa H. Catalyst/Feedstock Ratio Effect on FCC Using Different Catalysts Samples. Catalysts. 2019; 9(6):542. https://doi.org/10.3390/catal9060542
Chicago/Turabian StyleAlkhlel, Abdualkaber, and Hugo de Lasa. 2019. "Catalyst/Feedstock Ratio Effect on FCC Using Different Catalysts Samples" Catalysts 9, no. 6: 542. https://doi.org/10.3390/catal9060542
APA StyleAlkhlel, A., & de Lasa, H. (2019). Catalyst/Feedstock Ratio Effect on FCC Using Different Catalysts Samples. Catalysts, 9(6), 542. https://doi.org/10.3390/catal9060542