Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Phase Composition of NiO/NiTiO3 Composite Nanofibers
2.2. Morphology and Microstructure of the NiO/NiTiO3 Sample
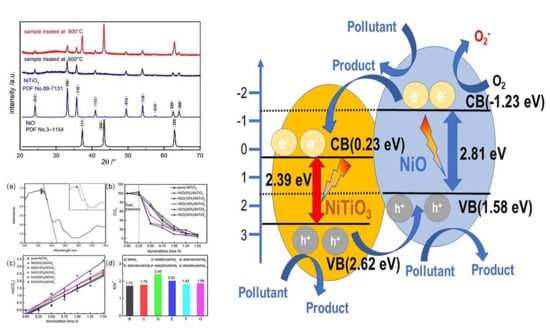
2.3. Photocatalytic Performance and Photocatalytic Mechanism
3. Materials and Methods
3.1. Experimental Reagent
3.2. Preparation of NiO/NiTiO3 Composite Nanofiber Membrane
3.3. Characterization and Performance Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, L.; Wang, J.; Liu, X.D.; Shu, X.Q.; Zhang, Z.H.; Wang, J. Fabrication of Z-scheme photocatalyst, Er3+:Y3Al5O12@NiGa2O4-MWCNTs-WO3, and visible-light photocatalytic activity for degradation of organic pollutant with simultaneous hydrogen evolution. Renew. Energy 2019, 138, 474–488. [Google Scholar] [CrossRef]
- Barhoumi, N.; Oturan, N.; Olvera-Vargas, H. Pyrite as a sustainable catalyst in electro-fenton process for improving oxidation of sulfamethazine. Kinetics, mechanism and toxicity assessment. Water Res. 2016, 94, 52. [Google Scholar] [CrossRef] [PubMed]
- Barrera, L.A.; Escobosa, A.C.; Nevarez, A.; Dominguez, N.; Banuelos, J.L.; Westerhoff, P.; Noveron, J.C. TiO2-carbon nanoporous composites prepared via ZnO nanoparticle-templated carbonization of glucose adsorb and photodegrade organic pollutants in water. J. Water Process Eng. 2019, 28, 331–338. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, X.X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Shao, C.L.; Li, X.H.; Zhang, L.; Xue, H.M.; Wang, C.H.; Liu, Y.C. Electrospun Nanofibers of ZnO-SnO2 Heterojunction with High Photocatalytic Activity. J. Phys. Chem. 2010, 114, 7920–7925. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.G. CdS/Graphene Nanocomposite Photocatalysts. Adv. Energy Mater. 2015, 5, 1500010. [Google Scholar] [CrossRef]
- Lei, Z.B.; You, W.S.; Liu, M.Y.; Zhou, G.H.; Takata, T.; Hara, M.; Domen, K.; Li, C. Photocatalytic water reduction under visible light on a novel ZnIn2S4 catalyst synthesized by hydrothermal method. Chem. Commun. 2003, 17, 2142–2143. [Google Scholar] [CrossRef]
- Hara, M.; Hitoki, G.; Takata, T.; Kondo, J.; Kobayashi, H.; Domen, K. TaON and Ta3N5 as new visible light driven photocatalysts. Catal. Today. 2003, 78, 555–560. [Google Scholar] [CrossRef]
- Zhu, G.L.; Lin, T.Q.; Lu, X.J.; Zhao, W.; Yang, C.Y.; Wang, Z.; Yin, H.; Liu, Z.Q.; Huang, F.Q.; Lin, J.H. Black brookite titania with high solar absorption and excellent photocatalytic performance. J. Mater. Chem. A 2003, 1, 9650–9653. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube-TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Zhang, H.C.; Huang, H.; Ming, H.; Li, H.T.; Zhang, L.L.; Liu, Y.; Kang, Z.H. Carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light. J. Mater. Chem. 2012, 22, 10501–10506. [Google Scholar] [CrossRef]
- Ju, J.F.; Chen, X.; Shi, Y.J.; Wu, D.H. Investigation of PdSn nanometals alloy supported on spherical TiO2 for methanol electro-oxidation. Powder Technol. 2013, 241, 1–6. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, M.; Chen, Q.G.; Fan, C.M.; Zhou, H.Y.; Xu, A.W. Novel onedimensional Bi2O3-Bi2WO6 p-n hierarchical heterojunction with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8517–8524. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, J.; Zhong, J.B.; Chen, J.F.; Li, J.Z.; Sun, S.Y. Charge separation behaviors of novel AgI/BiOI heterostructures with enhanced solar-photocatalytic performance. Curr. Appl. Phys. 2017, 17, 1202–1207. [Google Scholar] [CrossRef]
- Puddu, V.; Mokaya, R.; Puma, G.L. Novel one step hydrothermal synthesis of TiO2/WO3 nanocomposites with enhanced photocatalytic activity. Chem. Commun. 2007, 45, 4749–4751. [Google Scholar] [CrossRef]
- Teo, W.E.; Inai, R.; Ramakrishna, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002. [Google Scholar] [CrossRef]
- Yuan, P.H.; Fan, C.M.; Ding, G.Y.; Wang, Y.F.; Zhang, X.C. Preparation and photocatalytic properties of ilmenite NiTiO3 powders for degradation of humic acid in water. Int. J. Miner. Metall. Mater. 2012, 19, 372–376. [Google Scholar] [CrossRef]
- Hu, C.C.; Teng, H.S. Structural features of p-type semiconducting NiO as a co-catalyst for photocatalytic water splitting. J. Catal. 2010, 272, 1–8. [Google Scholar] [CrossRef]
- Moghiminia, S.; Farsi, H.; Raissi, H. Comparative optical and electrochemical studies of nanostructured NiTiO3 and NiTiO3-TiO2 prepared by a low temperature modified Sol-Gel route. Electrochim. Acta 2014, 132, 512–523. [Google Scholar] [CrossRef]
- Han, H.J.; Chao, S.J.; Yang, X.L.; Wang, X.B.; Wang, K.; Bai, Z.Y.; Yang, L. Ni nanoparticles embedded in N doped carbon nanotubes derived from a metal organic framework with improved performance for oxygen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 16149–16156. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z. Photocatalytic properties of gold/gold ion-modified titanium dioxide for wastewater treatment. Appl. Catal. A-Gen. 2002, 228, 5–27. [Google Scholar] [CrossRef]
- Hu, Y.; Tan, O.K.; Pan, J.S.; Yao, X. A new form of nanosized SrTiO3 material for near-human-body temperature oxygen sensing applications. J. Phys. Chem. B 2004, 108, 11214–11218. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.Y.; Du, S.C.; Meng, X.Y.; Tian, G.H.; Pan, K.; Wang, G.F.; Fu, H.G. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471–16476. [Google Scholar] [CrossRef]
- Yin, H.Y.; Zhu, J.J.; Chen, J.L.; Gong, J.Y.; Nie, Q.L. MOF-derived in situ growth of carbon nanotubes entangled Ni/NiO porous polyhedrons for high performance glucose sensor. Mater. Lett. 2018, 221, 267–270. [Google Scholar] [CrossRef]
- Pavithra, C.; Madhuri, W. Electrical and magnetic properties of NiTiO3 nanoparticles synthesized by the sol-gel synthesis method and microwave sintering. Mater. Chem. Phys. 2018, 211, 144–149. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Nithya, V.D.; Fathima, K.S.; Sanjeeviraja, C.; Selvan, G.K.; Arumugam, S.; Selvan, R.K. Investigations on the temperature dependent electrical and magnetic properties of NiTiO3 by molten salt synthesis. Mater. Res. Bull. 2012, 48, 1110–1116. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A.; Othman, I.; Ibrahim, I.A. Preparation of TiO2-ZSM-5 zeolite for photodegradation of EDTA. J. Mol. Catal. A-Chem. 2005, 238, 151–157. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Suc, N.V.; Chi, D.K. Removal of rhodamine B from aqueous solution via adsorption onto microwave-activated rice husk ash. J. Dispers. Sci. Technol. 2017, 38, 216–222. [Google Scholar] [CrossRef]
- Selvam, P.P.; Preethi, S.; Basakaralingam, P.; Thinakaran, N.; Sivasamy, A.; Sivanesan, S. Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. J. Hazard. Mater. 2008, 155, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, V.D.; Lopez-Sotelo, J.B.; Correa-Guimaraes, A.; Hernandez-Navarro, S.; Sanchez-Bascones, M.; Navas-Gracia, L.M.; Martin-Ramos, P.; Martin-Gil, J. Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J. Environ. Manag. 2015, 155, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.J.; Hu, S.W.; Jiang, W.; Liu, Y.P.; Zhou, Y.T.; Liu, Y.; Mo, L.Y. Hierarchical architectures of bismuth molybdate nanosheets onto nickel titanate nanofibers: Facile synthesis and efficient photocatalytic removal of tetracycline hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Arya, A.; Ningthoujam, R.S.; Tewari, R.; Rao, R.; Chalke, B.; Ayyub, P.; Tripathi, A.K.; et al. pn Heterojunctions in NiO:TiO2 composites with type-II band alignment assisting sunlight driven photocatalytic H-2 generation. Appl. Catal. B-Environ. 2018, 221, 443–458. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Electrospinning preparation of NiO/ZnO composite nanofibers for photodegradation of binary mixture of rhodamine B and methylene blue in aqueous solution: Central composite optimization. Appl. Organomet. Chem. 2018, 32, 6. [Google Scholar] [CrossRef]
- Najafian, H.; Manteghi, F.; Beshkar, F.; Salavati-Niasari, M. Enhanced photocatalytic activity of a novel NiO/Bi2O3/Bi3ClO4 nanocomposite for the degradation of azo dye pollutants under visible light irradiation. Sep. Purif. Technol. 2019, 209, 6–17. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Tsvetkov, M.; Spassov, T. Ammonia-evaporation-induced construction of three-dimensional NiO/g-C3N4 composite with enhanced adsorption and visible light-driven photocatalytic performance. Superlattices Microstruct. 2018, 119, 122–133. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Bai, X.; Wang, J.; Fang, M.; Wu, X.; Liu, Y.; Huang, Z.; Lao, C.-Y.; Min, X. Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films. Catalysts 2019, 9, 561. https://doi.org/10.3390/catal9060561
Yang B, Bai X, Wang J, Fang M, Wu X, Liu Y, Huang Z, Lao C-Y, Min X. Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films. Catalysts. 2019; 9(6):561. https://doi.org/10.3390/catal9060561
Chicago/Turabian StyleYang, Bozhi, Xuefeng Bai, Jiaxuan Wang, Minghao Fang, Xiaowen Wu, Yan’gai Liu, Zhaohui Huang, Cheng-Yen Lao, and Xin Min. 2019. "Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films" Catalysts 9, no. 6: 561. https://doi.org/10.3390/catal9060561
APA StyleYang, B., Bai, X., Wang, J., Fang, M., Wu, X., Liu, Y., Huang, Z., Lao, C. -Y., & Min, X. (2019). Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films. Catalysts, 9(6), 561. https://doi.org/10.3390/catal9060561