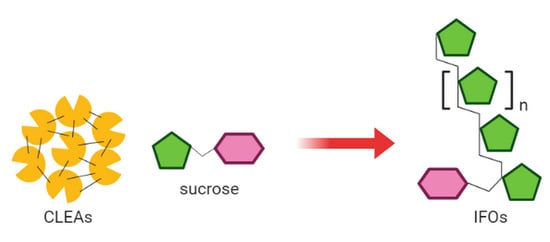
Preparation of Cross-Linked Enzyme Aggregates (CLEAs) of an Inulosucrase Mutant for the Enzymatic Synthesis of Inulin-Type Fructooligosaccharides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Immobilized Inulosucrase Mutant
2.2. Biochemical Characterization of Free and Immobilized R483A-LrInu
2.3. IFOs Synthesis Using Free and Immobilized R483A-LrInu
2.4. Operational Stability of R483A-LrInu CLEAs
3. Materials and Methods
3.1. Expression and Purification of Inulosucrase Mutant
3.2. Enzyme Activity Assay
3.3. Preparation of Cross-Linked Inulosucrase Aggregates
3.4. Biochemical Characterization of Free and CLEAs R483A-LrInu
3.5. Oligosaccharide Synthesis and Analysis
3.6. Operational Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Macfarlane, S.; Macfarlane, G.; Cummings, J.T. Prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006, 24, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Mabel, M.; Sangeetha, P.; Platel, K.; Srinivasan, K.; Prapulla, S. Physicochemical characterization of fructooligosaccharides and evaluation of their suitability as a potential sweetener for diabetics. Carbohydr. Res. 2008, 343, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Goosen, C.; Yuan, X.-L.; Van Munster, J.M.; Ram, A.F.; Van der Maarel, M.J.; Dijkhuizen, L. Molecular and biochemical characterization of a novel intracellular invertase from Aspergillus niger with transfructosylating activity. Eukaryot. Cell 2007, 6, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Nakane, A.; Kawate, A.; Hirayama, M. Molecular cloning and characterization of the fructooligosaccharide-producing β-fructofuranosidase gene from Aspergillus niger ATCC 20611. Biosci. Biotechnol. Biochem. 2001, 65, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Aguilar, C.N.; Rodrigues, L.R.; Teixeira, J.A. Fructooligosaccharides and β-fructofuranosidase production by Aspergillus japonicus immobilized on lignocellulosic materials. J. Mol. Catal. B Enzym. 2009, 59, 76–81. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Wang, J.-H.; Teng, D.; Zhang, F. Preparation of high-purity fructo-oligosaccharides by Aspergillus japonicus β-fructofuranosidase and successive cultivation with yeast. J. Agric. Food Chem. 2008, 56, 2805–2809. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, M.; Kimura, M.; Gozu, Y.; Sato, S.; Hirano, K.; Tochio, T.; Nishikawa, A.; Tonozuka, T. Crystal structure of a β-fructofuranosidase with high transfructosylation activity from Aspergillus kawachii. Biosci. Biotechnol. Biochem. 2017, 81, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Kurakake, M.; Masumoto, R.; Maguma, K.; Kamata, A.; Saito, E.; Ukita, N.; Komaki, T. Production of fructooligosaccharides by β-fructofuranosidases from Aspergillus oryzae KB. J. Agric. Food Chem. 2009, 58, 488–492. [Google Scholar] [CrossRef]
- Jiang, R.; Qiu, Y.; Huang, W.; Zhang, L.; Xue, F.; Ni, H.; Mei, D.; Gao, J.; Xu, H. One-step bioprocess of inulin to product inulo-oligosaccharides using bacillus subtilis secreting an extracellular endo-Inulinase. Appl. Biochem. Biotechnol. 2018, 187, 116–128. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, Y.J.; Song, S.K.; Yun, J.W. Production of inulo-oligosaccharides using endo-inulinase from a pseudomonas sp. Biotechnol. Lett. 1997, 19, 369–372. [Google Scholar]
- Ortiz-Soto, M.E.; Olivares-Illana, V.; López-Munguía, A. Biochemical properties of inulosucrase from Leuconostoc citreum CW28 used for inulin synthesis. Biocatal. Biotransformation 2004, 22, 275–281. [Google Scholar] [CrossRef]
- Van Hijum, S.; Van Geel-Schutten, G.; Rahaoui, H.; Van der Maarel, M.; Dijkhuizen, L. Characterization of a novel fructosyltransferase from Lactobacillus reuteri that synthesizes high-molecular-weight inulin and inulin oligosaccharides. Appl. Environ. Microbiol. 2002, 68, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Kralj, S.; Van der Maarel, M.J.; Dijkhuizen, L. The probiotic Lactobacillus johnsonii NCC 533 produces high-molecular-mass inulin from sucrose by using an inulosucrase enzyme. Appl. Environ. Microbiol. 2008, 74, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Kralj, S.; Pique, A.V.; Leemhuis, H.; Van der Maarel, M.J.; Dijkhuizen, L. Inulin and levan synthesis by probiotic Lactobacillus gasseri strains: Characterization of three novel fructansucrase enzymes and their fructan products. Microbiology 2010, 156, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Frasch, H.-J.; Van Leeuwen, S.S.; Dijkhuizen, L. Molecular and biochemical characteristics of the inulosucrase HugO from Streptomyces viridochromogenes DSM40736 (Tü494). Microbiology 2017, 163, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Kralj, S.; Leeflang, C.; Sierra, E.I.; Kempiński, B.; Alkan, V.; Kolkman, M. Synthesis of fructooligosaccharides (FosA) and inulin (InuO) by GH68 fructosyltransferases from Bacillus agaradhaerens strain WDG185. Carbohydr. Polym. 2018, 179, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Hu, C.; Yang, N.; Wang, Q.; Wang, J.; Pan, H.; Hu, Y.; Ruan, C. Enhanced activity and stability of industrial lipases immobilized onto spherelike bacterial cellulose. Int. J. Biol. Macromol. 2018, 109, 1174–1181. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzym. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef]
- Martins, M.; Jing, S.; Fu, J.; Cavaco-Paulo, A. Practical insights on enzyme stabilization. Crit. Rev. Biotechnol. 2018, 38, 335–350. [Google Scholar]
- Schoevaart, R.; Wolbers, M.; Golubovic, M.; Ottens, M.; Kieboom, A.; Van Rantwijk, F.; Van der Wielen, L.; Sheldon, R. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, G.; Cao, L.-C.; Ren, G.-H.; Kong, W.; Wang, S.-D.; Guo, G.-S.; Liu, Y.-H. Characterization of the cross-linked enzyme aggregates of a novel β-galactosidase, a potential catalyst for the synthesis of galacto-oligosaccharides. J. Agric. Food Chem. 2015, 63, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Soto, M.E.; Rudiño-Piñera, E.; Rodriguez-Alegria, M.E.; Munguia, A.L. Evaluation of cross-linked aggregates from purified Bacillus subtilis levansucrase mutants for transfructosylation reactions. BMC Biotechnol. 2009, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Charoenwongpaiboon, T.; Wangpaiboon, K.; Pichyangkura, R.; Prousoontorn, M.H. Highly porous core–shell chitosan beads with superb immobilization efficiency for Lactobacillus reuteri 121 inulosucrase and production of inulin-type fructooligosaccharides. RSC Adv. 2018, 8, 17008–17016. [Google Scholar] [CrossRef]
- Charoenwongpaiboon, T.; Sitthiyotha, T.; Na Ayutthaya, P.P.; Wangpaiboon, K.; Chunsrivirot, S.; Hengsakul Prousoontorn, M.; Pichyangkura, R. Modulation of fructooligosaccharide chain length and insight into the product binding motif of Lactobacillus reuteri 121 inulosucrase. Carbohydr. Polym. 2019, 209, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Charoenwongpaiboon, T.; Klaewkla, M.; Chunsrivirot, S.; Wangpaiboon, K.; Pichyangkura, R.; Field, R.A.; Prousoontorn, M.H. Rational re-design of Lactobacillus reuteri 121 inulosucrase for product chain length control. RSC Adv. 2019, 9, 14957–14965. [Google Scholar] [CrossRef]
- Yu, H.; Chen, H.; Wang, X.; Yang, Y.; Ching, C. Cross-linked enzyme aggregates (CLEAs) with controlled particles: Application to Candida rugosa lipase. J. Mol. Catal. B Enzym. 2006, 43, 124–127. [Google Scholar] [CrossRef]
- Aytar, B.S.; Bakir, U. Preparation of cross-linked tyrosinase aggregates. Process Biochem. 2008, 43, 125–131. [Google Scholar] [CrossRef]
- Xu, D.-Y.; Yang, Y.; Yang, Z. Activity and stability of cross-linked tyrosinase aggregates in aqueous and nonaqueous media. J. Biotechnol. 2011, 152, 30–36. [Google Scholar] [CrossRef]
- Sangeetha, K.; Abraham, T.E. Preparation and characterization of cross-linked enzyme aggregates (CLEA) of subtilisin for controlled release applications. Int. J. Biol. Macromol. 2008, 43, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Aksu, S.; Adao, P.; Pessoa, J.C.; Sheldon, R.A.; Arends, I.W. Vanadate substituted phytase: Immobilization, structural characterization and performance for sulfoxidations. J. Inorg. Biochem. 2008, 102, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Fazary, A.E.; Ismadji, S.; Ju, Y.-H. Biochemical studies on native and cross-linked aggregates of Aspergillus awamori feruloyl esterase. Int. J. Biol. Macromol. 2009, 44, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Ghodake, V.; Kate, A.; Samant, N.; Kumar, C.; Gadagkar, S. Preparation and characterization of cross-linked enzyme aggregates of Saccharomyces cerevisiae invertase. Aust. J. Basic Appl. Sci. 2010, 4, 4760–4765. [Google Scholar]
- Talekar, S.; Shah, V.; Patil, S.; Nimbalkar, M. Porous cross-linked enzyme aggregates (p-CLEAs) of Saccharomyces cerevisiae invertase. Catal. Sci. Technol. 2012, 2, 1575–1579. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Van Hijum, S.A.F.T.; Van der Maarel, M.J.E.C.; Dijkhuizen, L. Kinetic properties of an inulosucrase from Lactobacillus reuteri 121. FEBS Lett. 2003, 534, 207–210. [Google Scholar] [CrossRef]
- Karimpil, J.J.; Melo, J.; D’Souza, S. Hen egg white as a feeder protein for lipase immobilization. J. Mol. Catal. B Enzym. 2011, 71, 113–118. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Monsalve-Ledesma, L.; Ortega-Munoz, M.; Fernandez-Arrojo, L.; Ballesteros, A.; Santoyo-Gonzalez, F.; Plou, F. Vinyl sulfone-activated silica for efficient covalent immobilization of alkaline unstable enzymes: Application to levansucrase for fructooligosaccharide synthesis. RSC Adv. 2016, 6, 64175–64181. [Google Scholar] [CrossRef]
- Biedrzycka, E.; Bielecka, M. Prebiotic effectiveness of fructans of different degrees of polymerization. Trends Food Sci. Technol. 2004, 15, 170–175. [Google Scholar] [CrossRef]
- Ito, H.; Takemura, N.; Sonoyama, K.; Kawagishi, H.; Topping, D.L.; Conlon, M.A.; Morita, T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J. Agric. Food Chem. 2011, 59, 5771–5778. [Google Scholar] [CrossRef] [PubMed]
- Cha, X.; Han, S.; Yu, J.; Zhang, S.; Yu, S.; Fu, D.; Yao, M.; Zhang, L.; Feng, G. Inulin with a low degree of polymerization protects human umbilical vein endothelial cells from hypoxia/reoxygenation-induced injury. Carbohydr. Polym. 2019, 216, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenwongpaiboon, T.; Pichyangkura, R.; A. Field, R.; Prousoontorn, M.H. Preparation of Cross-Linked Enzyme Aggregates (CLEAs) of an Inulosucrase Mutant for the Enzymatic Synthesis of Inulin-Type Fructooligosaccharides. Catalysts 2019, 9, 641. https://doi.org/10.3390/catal9080641
Charoenwongpaiboon T, Pichyangkura R, A. Field R, Prousoontorn MH. Preparation of Cross-Linked Enzyme Aggregates (CLEAs) of an Inulosucrase Mutant for the Enzymatic Synthesis of Inulin-Type Fructooligosaccharides. Catalysts. 2019; 9(8):641. https://doi.org/10.3390/catal9080641
Chicago/Turabian StyleCharoenwongpaiboon, Thanapon, Rath Pichyangkura, Robert A. Field, and Manchumas Hengsakul Prousoontorn. 2019. "Preparation of Cross-Linked Enzyme Aggregates (CLEAs) of an Inulosucrase Mutant for the Enzymatic Synthesis of Inulin-Type Fructooligosaccharides" Catalysts 9, no. 8: 641. https://doi.org/10.3390/catal9080641
APA StyleCharoenwongpaiboon, T., Pichyangkura, R., A. Field, R., & Prousoontorn, M. H. (2019). Preparation of Cross-Linked Enzyme Aggregates (CLEAs) of an Inulosucrase Mutant for the Enzymatic Synthesis of Inulin-Type Fructooligosaccharides. Catalysts, 9(8), 641. https://doi.org/10.3390/catal9080641