Insights into the Pyrolysis Processes of Ce-MOFs for Preparing Highly Active Catalysts of Toluene Combustion
Abstract
:1. Introduction
2. Results and Discussion
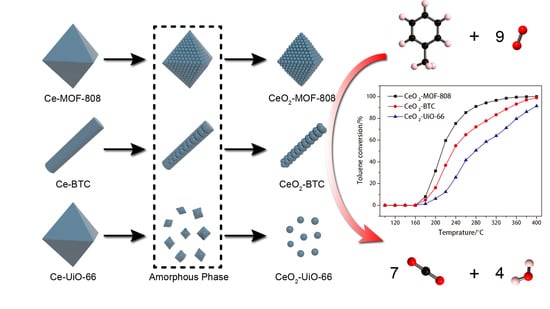
2.1. Preparation of Ce-MOFs and the Pyrolysis Process
2.1.1. Structural Analysis of Ce-MOFs
2.1.2. Changes in the Structures and Surface Morphologies upon Calcination
2.1.3. Structural Analysis of CeO2-MOFs
2.1.4. Proposed Mechanism of the Pyrolysis Processes
2.2. Characterization of CeO2-MOFs
2.2.1. Surface Composition of CeO2-MOFs
2.2.2. Oxygen Storage Capacity (OSC) and Reducibility of CeO2-MOFs
2.3. Catalytic Performance Evaluation of CeO2-MOFs on Toluene Oxidation
2.3.1. Catalytic Performance of the CeO2-MOFs for Toluene Oxidation
2.3.2. Stability Tests of CeO2-MOFs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Three MOFs
3.2.1. Synthesis of Ce-MOF-808
3.2.2. Synthesis of Ce-BTC
3.2.3. Synthesis of Ce-UiO-66
3.3. Synthesis of CeO2-MOFs
3.4. Characterization
3.5. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, X.L.; Ding, A.J.; Poon, C.N.; Lam, K.S.; Li, Y.S.; Chan, L.Y.; Anson, M. Increasing surface ozone concentrations in the background atmosphere of Southern China, 1994–2007. Atmos. Chem. Phys. 2009, 9, 6217–6227. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Cheng, N. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624–2639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Zhang, S.; You, J.; Kennes, C.; Cheng, Z.; Ye, J.; Chen, D.; Chen, J.; Wang, L. Current advances of VOCs degradation by bioelectrochemical systems: A review. Chem. Eng. J. 2018, 334, 2625–2637. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Temerev, V.L.; Vedyagin, A.A.; Afonasenko, T.N.; Iost, K.N.; Kotolevich, Y.S.; Baltakhinov, V.P.; Tsyrulnikov, P.G. Effect of Ag loading on the adsorption/desorption properties of ZSM-5 towards toluene. React. Kinet. Mech. Catal. 2016, 119, 629–640. [Google Scholar] [CrossRef]
- Behar, S.; Gomez-Mendoza, N.-A.; Gomez-Garcia, M.-A.; Swierczynski, D.; Quignard, F.; Tanchoux, N. Study and modelling of kinetics of the oxidation of VOC catalyzed by nanosized Cu-Mn spinels prepared via an alginate route. Appl. Catal. A Gen. 2015, 504, 203–210. [Google Scholar] [CrossRef]
- Carrillo, A.M.; Carriazo, J.G. Cu and Co oxides supported on halloysite for the total oxidation of toluene. Appl. Catal. B Environ. 2015, 164, 443–452. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sanchez-Royo, J.F.; Murillo, R.; Maria Mastral, A.; Aranda, A.; Vazquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Schilling, C.; Hess, C. Elucidating the Role of Support Oxygen in the Water-Gas Shift Reaction over Ceria-Supported Gold Catalysts Using Operando Spectroscopy. ACS Catal. 2019, 9, 1159–1171. [Google Scholar] [CrossRef]
- Huang, N.; Geng, Y.; Xiong, S.; Huang, X.; Kong, L.; Yang, S.; Peng, Y.; Chen, J.; Li, J. The promotion effect of ceria on high vanadia loading NH3-SCR catalysts. Catal. Commun. 2019, 121, 84–88. [Google Scholar] [CrossRef]
- Zhao, P.; Qin, F.; Huang, Z.; Sun, C.; Shen, W.; Xu, H. MOF-derived hollow porous Ni/CeO2 octahedron with high efficiency for N2O decomposition. Chem. Eng. J. 2018, 349, 72–81. [Google Scholar] [CrossRef]
- Feng, Z.; Ren, Q.; Peng, R.; Mo, S.; Zhang, M.; Fu, M.; Chen, L.; Ye, D. Effect of CeO2 morphologies on toluene catalytic combustion. Catal. Today 2019, 332, 177–182. [Google Scholar] [CrossRef]
- Manuel Lopez, J.; Gilbank, A.L.; Garcia, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174, 403–412. [Google Scholar] [CrossRef]
- Hu, F.; Chen, J.; Peng, Y.; Song, H.; Li, K.; Li, J. Novel nanowire self-assembled hierarchical CeO2 microspheres for low temperature toluene catalytic combustion. Chem. Eng. J. 2018, 331, 425–434. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Wu, X.; Zhan, Y.; Wang, X.; Au, C.-T.; Jiang, L. Synthesis of Co-Mn oxides with double-shelled nanocages for low-temperature toluene combustion. Catal. Sci. Technol. 2018, 8, 4494–4502. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Zuo, J.; Feng, X.; Wang, X.; Zhang, T.; Zhang, K.; Jiang, L. Insights into the high performance of Mn-Co oxides derived from metal organic frameworks for total toluene oxidation. J. Hazard. Mater. 2018, 349, 119–127. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Guo, S.; Wang, Y.; Han, X.; Bai, Y. Efficient oxidation of o-xylene over CeO2 catalyst prepared from a Ce-MOF template: The promotion of K+ embedding substitution. Mol. Catal. 2017, 436, 120–127. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Lammert, M.; Glissmann, C.; Reinsch, H.; Stock, N. Synthesis and Characterization of New Ce(IV)-MOFs Exhibiting Various Framework Topologies. Cryst. Growth Des. 2017, 17, 1125–1131. [Google Scholar] [CrossRef]
- Liu, K.; You, H.; Jia, G.; Zheng, Y.; Huang, Y.; Song, Y.; Yang, M.; Zhang, L.; Zhang, H. Hierarchically Nanostructured Coordination Polymer: Facile and Rapid Fabrication and Tunable Morphologies. Cryst. Growth Des. 2010, 10, 790–797. [Google Scholar] [CrossRef]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; De Vos, D.; Stock, N. Cerium-based metal organic frameworks with UiO-66 architecture: Synthesis, properties and redox catalytic activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Z.; Zhao, W.; Gao, D.; Li, C.; Ye, C.; Sun, G. Design of Porous/Hollow Structured Ceria by Partial Thermal Decomposition of Ce-MOF and Selective Etching. ACS Appl. Mater. Interfaces 2017, 9, 39594–39601. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Belyaeva, E.V.; Fitch, A.N.; Chernyshev, V.V.; Klyamkin, S.N.; Kustov, L.M. Synthesis and Structural Characterization of a Series of Novel Zn(II)-based MOFs with Pyridine-2, 5-dicarboxylate Linkers. Cryst. Growth Des. 2013, 13, 5305–5315. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Lin, M.; Ma, X.; Ge, M. Enhancement effect of acid treatment on Mn2O3 catalyst for toluene oxidation. Catal. Today 2019, 327, 254–261. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Lefferts, L.; Mojet, B.L. Defect Chemistry of Ceria Nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Z.; Li, J.; Ma, Y.; Qu, Y. Surface engineering on CeO2 nanorods by chemical redox etching and their enhanced catalytic activity for CO oxidation. Nanoscale 2015, 7, 11686–11691. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M., III; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Lu, H.-F.; Zhou, Y.; Han, W.-F.; Huang, H.-F.; Chen, Y.-F. Promoting effect of ZrO2 carrier on activity and thermal stability of CeO2-based oxides catalysts for toluene combustion. Appl. Catal. A Gen. 2013, 464, 101–108. [Google Scholar] [CrossRef]
- He, H.; Lin, X.; Li, S.; Wu, Z.; Gao, J.; Wu, J.; Wen, W.; Ye, D.; Fu, M. The key surface species and oxygen vacancies in MnOx(0.4)-CeO2 toward repeated soot oxidation. Appl. Catal. B Environ. 2018, 223, 134–142. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Wang, J.; Xiao, H.; Chen, B.; Peng, R.; Fu, M.; Chen, L.; Ye, D.; Wen, W. Methanol plasma-catalytic oxidation over CeO2 catalysts: Effect of ceria morphology and reaction mechanism. Chem. Eng. J. 2019, 369, 233–244. [Google Scholar] [CrossRef]
- Alikin, E.A.; Vedyagin, A.A. High Temperature Interaction of Rhodium with Oxygen Storage Component in Three-Way Catalysts. Top. Catal. 2016, 59, 1033–1038. [Google Scholar] [CrossRef]










| Specific Surface Area (m2/g) | Micropore Area (m2/g) | Micropore Volume (cm3/g) | Micropore Width (nm) | |
|---|---|---|---|---|
| Ce-MOF-808 | 469.4 | 350.4 | 0.182 | 1.948 |
| Ce-BTC | 28.9 | - | - | - |
| Ce-UiO-66 | 425.0 | 234.3 | 0.126 | 1.022 |
| Particle Size (nm) a | Particle Size (nm) b | Specific Surface Area (m2/g) | ID/IF2g | Ce3+/(Ce3+ + Ce4+) (%) | Osur/Olatt (%) | |
|---|---|---|---|---|---|---|
| CeO2-MOF-808 | 5.2 | 6.7 | 96.6 | 0.035 | 25.4 | 46.8 |
| CeO2-BTC | 9.7 | 9.8 | 71.5 | 0.026 | 24.4 | 36.8 |
| CeO2-UiO-66 | 10.6 | 11.1 | 38.8 | 0.018 | 19.1 | 30.2 |
| Oxides | Precursor | T50 | T90 | Toluene Concentration | GHSV | Reference |
|---|---|---|---|---|---|---|
| CeO2 | Ce-MOF-808 | 218 | 278 | 1000 | 60,000 | This work |
| CeO2 | Ce-BTC | 232 | 343 | 1000 | 60,000 | This work |
| CeO2 | Ce-UiO-66 | 280 | 392 | 1000 | 60,000 | This work |
| CeO2 | Ce-BTC | 211 | 223 | 1000 | 20,000 | Reference [22] |
| 233 | 251 | 120,000 | Reference [22] | |||
| Co-Mn | Co-Mn-MOF | 226 | 240 | 500 | 96,000 | Reference [20] |
| Co-Mn | ZIF-74 | 232 | 248 | 1000 | 60,000 | Reference [19] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Li, X.; Sun, C.; Huang, Z.; Xu, H.; Shen, W. Insights into the Pyrolysis Processes of Ce-MOFs for Preparing Highly Active Catalysts of Toluene Combustion. Catalysts 2019, 9, 682. https://doi.org/10.3390/catal9080682
Sun W, Li X, Sun C, Huang Z, Xu H, Shen W. Insights into the Pyrolysis Processes of Ce-MOFs for Preparing Highly Active Catalysts of Toluene Combustion. Catalysts. 2019; 9(8):682. https://doi.org/10.3390/catal9080682
Chicago/Turabian StyleSun, Wenjie, Xiaomin Li, Chao Sun, Zhen Huang, Hualong Xu, and Wei Shen. 2019. "Insights into the Pyrolysis Processes of Ce-MOFs for Preparing Highly Active Catalysts of Toluene Combustion" Catalysts 9, no. 8: 682. https://doi.org/10.3390/catal9080682
APA StyleSun, W., Li, X., Sun, C., Huang, Z., Xu, H., & Shen, W. (2019). Insights into the Pyrolysis Processes of Ce-MOFs for Preparing Highly Active Catalysts of Toluene Combustion. Catalysts, 9(8), 682. https://doi.org/10.3390/catal9080682