Mild Preoxidation Treatment of Pt/TiO2 Catalyst and Its Enhanced Low Temperature Formaldehyde Decomposition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formaldehyde Decomposition Performancs
2.2. Structural Analyses
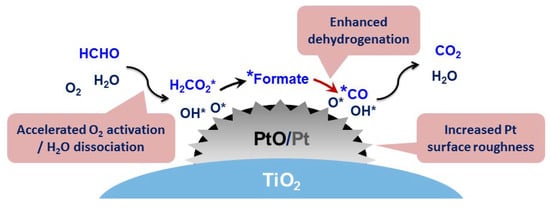
2.3. Structure-Performance Relationship Discussion
3. Materials and Methods
3.1. Chemicals and Synthesis
3.2. Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bianchi, F.; Careri, M.; Musci, M.; Mangia, A. Fish and food safety: Determination of formaldehyde in 12 fish species by SPME extraction and GC–MS analysis. Food Chem. 2007, 100, 1049–1053. [Google Scholar] [CrossRef]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H.; Tanaka, K.-I. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Zhai, Y.; Ariga, H.; Yi, N.; Liu, Y.; Asakura, K.; Flytzani-Stephanopoulos, M.; He, H. Alkali-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures. Angew. Chem. Int. Ed. 2012, 51, 9628–9632. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tran, T.P.; Vo, D.V.; Sakurai, M.; Kameyama, H. Design of novel Pt-structured catalyst on anodic aluminum support for VOC’s catalytic combustion. Appl. Catal. A Gen. 2008, 350, 150–156. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; He, H.; Zhang, J.; Chen, M. Influence of alkali metals on Pd/TiO2 catalysts for catalytic oxidation of formaldehyde at room temperature. Catal. Sci. Technol. 2016, 6, 2289–2295. [Google Scholar] [CrossRef]
- Sun, X.; Lin, J.; Guan, H.; Li, L.; Sun, L.; Wang, Y.; Miao, S.; Su, Y.; Wang, X. Complete oxidation of formaldehyde over TiO2 supported subnanometer Rh catalyst at ambient temperature. Appl. Catal. B Environ. 2018, 226, 575–584. [Google Scholar] [CrossRef]
- Zhu, L.; Jacob, D.J.; Keutsch, F.N.; Mickley, L.J.; Scheffe, R.; Strum, M.; González Abad, G.; Chance, K.; Yang, K.; Rappenglück, B. Formaldehyde (HCHO) as a hazardous air pollutant: Mapping surface air concentrations from satellite and inferring cancer risks in the United States. Environ. Sci. Technol. 2017, 51, 5650–5657. [Google Scholar] [CrossRef]
- Al Soubaihi, R.; Saoud, K.; Dutta, J. Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Jaroniec, M.; Tao, F.F. Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 2016, 6, 3649–3669. [Google Scholar] [CrossRef]
- Guo, J.; Lin, C.; Jiang, C.; Zhang, P. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Li, J.; Hao, J. Progress in research on catalysts for catalytic oxidation of formaldehyde. Chin. J. Catal. 2016, 37, 102–122. [Google Scholar] [CrossRef]
- Huang, H.; Hu, P.; Huang, H.; Chen, J.; Ye, X.; Leung, D.Y. Highly dispersed and active supported Pt nanoparticles for gaseous formaldehyde oxidation: Influence of particle size. Chem. Eng. J. 2014, 252, 320–326. [Google Scholar] [CrossRef]
- An, N.; Zhang, W.; Yuan, X.; Pan, B.; Liu, G.; Jia, M.; Yan, W.; Zhang, W. Catalytic oxidation of formaldehyde over different silica supported platinum catalysts. Chem. Eng. J. 2013, 215, 1–6. [Google Scholar] [CrossRef]
- An, N.; Yu, Q.; Liu, G.; Li, S.; Jia, M.; Zhang, W. Complete oxidation of formaldehyde at ambient temperature over supported Pt/Fe2O3 catalysts prepared by colloid-deposition method. J. Hazard. Mater. 2011, 186, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Leung, D.Y.; Ye, D. Effect of reduction treatment on structural properties of TiO2 supported Pt nanoparticles and their catalytic activity for formaldehyde oxidation. J. Mater. Chem. 2011, 21, 9647–9652. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Highly active mesoporous ferrihydrite supported Pt catalyst for formaldehyde removal at room temperature. Environ. Sci. Technol. 2015, 49, 6637–6644. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, S. Correlation between microstructure and performance of Pt/TiO2 catalysts for formaldehyde catalytic oxidation at ambient temperature: effects of hydrogen pretreatment. J. Phys. Chem. C 2007, 111, 9897–9904. [Google Scholar] [CrossRef]
- Xu, F.; Le, Y.; Cheng, B.; Jiang, C. Effect of calcination temperature on formaldehyde oxidation performance of Pt/TiO2 nanofiber composite at room temperature. Appl. Surf. Sci. 2017, 426, 333–341. [Google Scholar] [CrossRef]
- Li, L.; Yue, H.; Zhang, S.; Huang, Y.; Zhang, W.; Wu, P.; Ji, Y.; Huo, F. Solving the Water Hypersensitive Challenge of Sulfated Solid Superacid in Acid-Catalyzed Reactions. ACS Appl. Mater. Interfaces 2019, 11, 9919–9924. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chen, T.-C.; Chen, Y.-C.; Lee, J.-F.; Chen, C.-S. Formaldehyde oxidation on silica-supported Pt catalysts: The influence of thermal pretreatments on particle formation and on oxidation mechanism. J. Catal. 2017, 355, 87–100. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Ma, J.; Chen, M.; Deng, H.; He, H. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation. Appl. Catal. B Environ. 2017, 217, 560–569. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Rong, S.; Wang, H.; Zhang, P. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature. Appl. Catal. B Environ. 2017, 211, 212–221. [Google Scholar] [CrossRef]
- Alayon, E.; Singh, J.; Nachtegaal, M.; Harfouche, M.; van Bokhoven, J.A. On highly active partially oxidized platinum in carbon monoxide oxidation over supported platinum catalysts. J. Catal. 2009, 263, 228–238. [Google Scholar] [CrossRef]
- Ke, J.; Zhu, W.; Jiang, Y.; Si, R.; Wang, Y.-J.; Li, S.-C.; Jin, C.; Liu, H.; Song, W.-G.; Yan, C.-H. Strong Local Coordination Structure Effects on Subnanometer PtOx Clusters over CeO2 Nanowires Probed by Low-Temperature CO Oxidation. ACS Catal. 2015, 5, 5164–5173. [Google Scholar] [CrossRef]
- Parayil, S.K.; Kibombo, H.S.; Wu, C.-M.; Peng, R.; Kindle, T.; Mishra, S.; Ahrenkiel, S.P.; Baltrusaitis, J.; Dimitrijevic, N.M.; Rajh, T. Synthesis-dependent oxidation state of platinum on TiO2 and their influences on the solar simulated photocatalytic hydrogen production from water. J. Phys. Chem. C 2013, 117, 16850–16862. [Google Scholar] [CrossRef]
- Kuhaudomlap, S.; Mekasuwandumrong, O.; Praserthdam, P.; Fujita, S.-I.; Arai, M.; Panpranot, J. The H2-Treated TiO2 Supported Pt Catalysts Prepared by Strong Electrostatic Adsorption for Liquid-Phase Selective Hydrogenation. Catalysts 2018, 8, 87. [Google Scholar] [CrossRef]
- Li, M.; Song, J.; Yue, F.; Pan, F.; Yan, W.; Hua, Z.; Li, L.; Yang, Z.; Li, L.; Wen, G. Complete Hydrodesulfurization of Dibenzothiophene via Direct Desulfurization Pathway over Mesoporous TiO2-Supported NiMo Catalyst Incorporated with Potassium. Catalysts 2019, 9, 448. [Google Scholar] [CrossRef]
- Li, L.; Yue, H.; Ji, T.; Li, W.; Zhao, X.; Wang, L.; She, J.; Gu, X.; Li, X. Novel mesoporous TiO2(B) whisker-supported sulfated solid superacid with unique acid characteristics and catalytic performances. Appl. Catal. A Gen. 2019, 574, 25–32. [Google Scholar] [CrossRef]
- Huizinga, T.; Van Grondelle, J.; Prins, R. A temperature programmed reduction study of Pt on Al2O3 and TiO2. Appl. catal. 1984, 10, 199–213. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, M.; Lobban, L.L.; Mallinson, R.G. Structural effects of Na promotion for high water gas shift activity on Pt–Na/TiO2. J. Catal. 2011, 278, 123–132. [Google Scholar] [CrossRef]
- Wang, L.; Sakurai, M.; Kameyama, H. Study of catalytic decomposition of formaldehyde on Pt/TiO2 alumite catalyst at ambient temperature. J. Hazard. Mater. 2009, 167, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Epling, W.S.; Cheekatamarla, P.K.; Lane, A.M. Reaction and surface characterization studies of titania-supported Co, Pt and Co/Pt catalysts for the selective oxidation of CO in H2-containing streams. Chem. Eng. J. 2003, 93, 61–68. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y. Complete elimination of indoor formaldehyde over supported Pt catalysts with extremely low Pt content at ambient temperature. J. Catal. 2011, 280, 60–67. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Cai, Q.; Mo, G.; Jiang, L.; Zhang, K.; Chen, Z.; Wu, Z.; Pan, W. In situ SAXS study on size changes of platinum nanoparticles with temperature. Eur. Phys. J. B 2008, 65, 57–64. [Google Scholar] [CrossRef]
- Ono, L.; Yuan, B.; Heinrich, H.; Cuenya, B.R. Formation and thermal stability of platinum oxides on size-selected platinum nanoparticles: support effects. J. Phys. Chem. C 2010, 114, 22119–22133. [Google Scholar] [CrossRef]
- Vovk, E.I.; Kalinkin, A.V.; Smirnov, M.Y.; Klembovskii, I.O.; Bukhtiyarov, V.I. XPS study of stability and reactivity of oxidized Pt nanoparticles supported on TiO2. J. Phys. Chem. C 2017, 121, 17297–17304. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Sepúlveda-Escribano, A.; Anderson, J.A.; Rodríguez-Reinoso, F. Spectroscopic and microcalorimetric study of a TiO2-supported platinum catalyst. Phys. Chem. Chem. Phys. 2009, 11, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulou, P.; Kondarides, D.I. Effects of alkali additives on the physicochemical characteristics and chemisorptive properties of Pt/TiO2 catalysts. J. Catal. 2008, 260, 141–149. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Christodoulakis, A.; Kondarides, D.; Boghosian, S. Particle size effects on the reducibility of titanium dioxide and its relation to the water–gas shift activity of Pt/TiO2 catalysts. J. Catal. 2006, 240, 114–125. [Google Scholar] [CrossRef]
- Hayden, B.; Kretzschmar, K.; Bradshaw, A.; Greenler, R. An infrared study of the adsorption of CO on a stepped platinum surface. Surf. Sci. 1985, 149, 394–406. [Google Scholar] [CrossRef]
- Kale, M.J.; Christopher, P. Utilizing quantitative in situ FTIR spectroscopy to identify well-coordinated Pt atoms as the active site for CO oxidation on Al2O3-supported Pt catalysts. ACS Catal. 2016, 6, 5599–5609. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, M.; Li, M.; Lee, S.; Flytzani-Stephanopoulos, M. Low-Coordinated Pd Catalysts Supported on Zn1Zr1Ox Composite Oxides for Selective Methanol Steam Reforming. Appl. Catal. A Gen. 2019, 580, 81–92. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, C.; Sun, X.; Deng, S.; Zhuang, G.; Zhong, X.; Wei, Z.; Yao, Z.; Wang, J.-g. Multiscale Simulation of Morphology Evolution of Supported Pt Nanoparticles via Interfacial Control. Langmuir 2019, 35, 6393–6402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H. A comparative study of TiO2 supported noble metal catalysts for the oxidation of formaldehyde at room temperature. Catalysis Today 2007, 126, 345–350. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.; Guo, W.; Zhu, H.; Li, M.; Zhao, L.; Li, Y.; Shan, H. Formaldehyde oxidation on the Pt/TiO2(101) surface: A DFT investigation. J. Organomet. Chem. 2012, 704, 38–48. [Google Scholar] [CrossRef]
- Mao, C.-F.; Vannice, M.A. Formaldehyde oxidation over Ag catalysts. J. Catal. 1995, 154, 230–244. [Google Scholar] [CrossRef]








| Sample | Crystal Size a (nm) | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|---|
| Pt/TiO2 | 19.6 | 46.3 | 0.215 | 14.1 |
| Pt/TiO2-O100 | 20.0 | 43.0 | 0.215 | 14.7 |
| Pt/TiO2-O200 | 19.8 | 44.1 | 0.219 | 14.5 |
| Pt/TiO2-O300 | 19.2 | 46.8 | 0.218 | 13.9 |
| Catalyst | BE of Pt4f7/2 eV | Ptδ+/(Pt0 + Ptδ+) % | BE of O1s eV | OII/OI | OH−/OI |
|---|---|---|---|---|---|
| Pt/TiO2 | 70.9 | 30.1 | 529.7 | 0.065 | <0.001 |
| Pt/TiO2-O100 | 71.1 | 40.3 | 529.5 | 0.121 | 0.066 |
| Pt/TiO2-O200 | 70.7 | 40.4 | 529.6 | 0.148 | 0.042 |
| Pt/TiO2-O300 | 70.8 | 57.3 | 529.7 | 0.116 | 0.018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Wang, L.; Li, L.; Zhao, X.; Chen, Y.; Hua, Z.; Li, X.; Gu, X.; Li, L. Mild Preoxidation Treatment of Pt/TiO2 Catalyst and Its Enhanced Low Temperature Formaldehyde Decomposition. Catalysts 2019, 9, 694. https://doi.org/10.3390/catal9080694
Shi K, Wang L, Li L, Zhao X, Chen Y, Hua Z, Li X, Gu X, Li L. Mild Preoxidation Treatment of Pt/TiO2 Catalyst and Its Enhanced Low Temperature Formaldehyde Decomposition. Catalysts. 2019; 9(8):694. https://doi.org/10.3390/catal9080694
Chicago/Turabian StyleShi, Kangzhong, Lei Wang, Long Li, Xuejuan Zhao, Yuanyuan Chen, Zelin Hua, Xiaobao Li, Xiaoli Gu, and Licheng Li. 2019. "Mild Preoxidation Treatment of Pt/TiO2 Catalyst and Its Enhanced Low Temperature Formaldehyde Decomposition" Catalysts 9, no. 8: 694. https://doi.org/10.3390/catal9080694
APA StyleShi, K., Wang, L., Li, L., Zhao, X., Chen, Y., Hua, Z., Li, X., Gu, X., & Li, L. (2019). Mild Preoxidation Treatment of Pt/TiO2 Catalyst and Its Enhanced Low Temperature Formaldehyde Decomposition. Catalysts, 9(8), 694. https://doi.org/10.3390/catal9080694