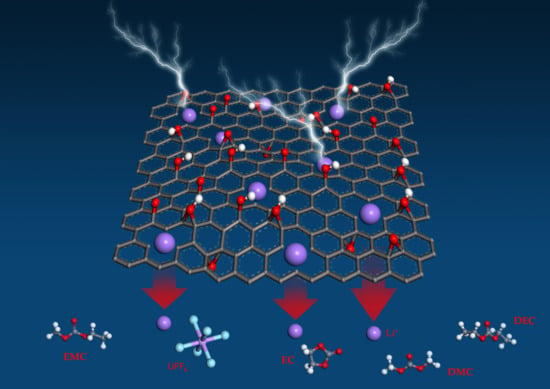
Experimental and Modeling Analysis of Holey Graphene Electrodes for High-Power-Density Li-Ion Batteries
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of Pristine and Holey Graphene Samples
2.2. Characterizations
2.3. Electrochemical Measurements
3. Simulations
3.1. Simulated Systems
3.2. Simulation Method
4. Results and Discussion
4.1. Experimental Results
4.2. Simulation Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaskhedikar, N.A.; Maier, J. Lithium Storage in Carbon Nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.K.E.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Uthaisar, C.; Barone, V. Edge effects on the characteristics of Li diffusion in graphene. Nano Lett. 2010, 10, 2838–2842. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.; Sethuraman, V.A.; Hardwick, L.J.; Hinuma, Y.; Meng, Y.S.; van der Ven, A.; Srinivasan, V.; Kostecki, R.; Ceder, G. Lithium Diffusion in Graphitic Carbon. Phys. Chem. Lett. 2010, 1, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, D.; Too, C.O.; Wallace, G.G. Electrochemical Properties of Graphene Paper Electrodes Used in Lithium Batteries. Chem. Mater. 2009, 21, 2604–2606. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Abouimrane, A.; Compton, O.C.; Amine, K.; Nguyen, S.T. Non-Annealed Graphene Paper as a Binder-Free Anode for Lithium-Ion Batteries. J. Phys. Chem. C 2010, 114, 12800–12804. [Google Scholar] [CrossRef]
- Xi, K.; Kidambi, P.R.; Chen, R.; Gao, C.; Peng, X.; Ducati, C.; Hofmann, S.; Kumar, R.V. Binder free three-dimensional sulphur/few-layer graphene foam cathode with enhanced high-rate capability for rechargeable lithium sulphur batteries. Nanoscale 2014, 6, 5746–5753. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhao, X.; Wu, J.; Jang, H.D.; Kung, H.H.; Huang, J. Crumpled Graphene-Encapsulated Si Nanoparticles for Lithium Ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1824–1829. [Google Scholar] [CrossRef]
- Luo, J.; Jang, H.D.; Huang, J. Effect of Sheet Morphology on the Scalability of Graphene-Based Ultracapacitors. ACS Nano 2013, 7, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Wen, Z.; Kim, H.; Lu, G.; Hurley, P.; Chen, J. A general approach to one-pot fabrication of crumpled graphene-based nanohybrids for energy applications. ACS Nano 2012, 6, 7505–7513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, X.; Braun, P.V. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotechnol. 2011, 6, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Pu, N.-W.; Wu, P.-J.; Peng, Y.-Y.; Shih, C.-N.; Chen, C.-Y.; Liu, Y.-M.; Ger, M.-D. Performance improvement of lithium ion batteries using magnetite–graphene nanocomposite anode materials synthesized by a microwave-assisted method. Microelectron. Eng. 2015, 138, 47–51. [Google Scholar] [CrossRef]
- Fan, L.; Li, X.; Yan, B.; Li, X.; Xiong, D.; Li, D.; Xu, H.; Zhang, X.; Sun, X. Amorphous SnO2/graphene aerogel nanocomposites harvesting superior anode performance for lithium energy storage. Appl. Energy 2016, 175, 529–535. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Liu, Y.-M.; Chang, J.-K.; Wu, C.-H.; Ger, M.-D.; Pu, N.-W.; Chang, C.-L. A facile approach to produce holey graphene and its application in supercapacitors. Carbon 2015, 81, 347–356. [Google Scholar] [CrossRef]
- Wu, C.-H.; Pu, N.-W.; Liu, Y.-M.; Chen, C.-Y.; Peng, Y.-Y.; Cheng, T.-Y.; Lin, M.-H.; Ger, M.-D. Improving rate capability of lithium-ion batteries using holey graphene as the anode material. J. Taiwan Inst. Chem. Eng. 2017, 80, 511–517. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.Y.; Zhao, Z.; Lin, Z.; Lee, C.; Xu, X.; Wang, C.; Huang, Y.; Shakir, M.I.; Duan, X. Solution Processable Holey Graphene Oxide and Its Derived Macrostructures for High-Performance Supercapacitors. Nano Lett. 2015, 15, 4605–4610. [Google Scholar] [CrossRef]
- Ding, B.; Yuan, C.; Shen, L.; Xu, G.; Nie, P.; Lai, Q.; Zhang, X. Chemically tailoring the nanostructure of graphenenanosheets to confine sulfur for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2013, 1, 1096–1101. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Meng, Q.-H.; Li, J.-T.; Zhu, J.-H.; Cui, Y.; Han, B.-H. High surface area porous carbons produced by steam activation of graphene aerogels. J. Mater. Chem. A 2014, 2, 9891–9898. [Google Scholar] [CrossRef]
- Vargas, O.; Caballero, A.; Morales, J.; Elia, G.A.; Scrosati, B.; Hassoun, J. Electrochemical performance of a graphene nanosheets anode in a high voltage lithium-ion cell. Phys.Chem. Chem. Phys. 2013, 15, 20444–20446. [Google Scholar] [CrossRef] [PubMed]
- Vargas, O.; Caballero, A.; Morales, J.; Rodríguez-Castellón, E. Contribution to the Understanding of Capacity Fading in Graphene Nanosheets Acting as an Anode in Full Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An advanced lithium-ion battery based on a graphene anode and a lithium iron phosphate cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, O.; Caballero, A.; Morales, J. Deficiencies of Chemically Reduced Graphene as Electrode in Full Li-Ion Cells. Electrochim. Acta 2015, 165, 365–371. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Theoretical studies on cosolvation of Li ion and solvent reductive decomposition in binary mixtures of aliphatic carbonates. Int. J. Quantum Chem. 2005, 102, 724–733. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase—The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Phys.Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Verma, P.M.P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide. Phys. Chem. B 2006, 110, 8535–8538. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.R.; Chen, C.L.; Tseng, Y.H.; Chien, C.T.C.; Liu, C.W.; Tai, C.C.; Liu, M.H. Graphene Wrinkles affect electronic transport in nanocomposites: Insight from molecular dynamics simulations. J. Mol. Graph. Model. 2019, 92, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-R.; Chuang, P.-H.; Chen, C.-L. Molecular-dynamics calculation of the thermal conduction in phase change materials of graphene paraffin nanocomposites. Int. J. Heat Mass Transf. 2015, 91, 45–51. [Google Scholar] [CrossRef]
- Rappi, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Ma, L.-P.; Wu, Z.-S.; Li, J.; Wu, E.-D.; Ren, W.-C.; Cheng, H.-M. Hydrogen adsorption behavior of graphene above critical temperature. Int. J. Hydrog. Energy 2009, 34, 2329–2332. [Google Scholar] [CrossRef]
- Huang, C.C.; Pu, N.W.; Wang, C.A.; Huang, J.; Sung, Y.; Ger, M.D. Hydrogen storage in graphene decorated with Pd and Pt nano-particles using an electroless deposition technique. Sep. Purif. Technol. 2011, 82, 210–215. [Google Scholar] [CrossRef]
- Tao, H.-C.; Fan, L.-Z.; Yang, X.; Qu, X. In situ synthesis of TiO2-graphene nanosheets composites as anode materials for high-power lithium ion batteries. Electrochim. Acta 2012, 69, 328–333. [Google Scholar] [CrossRef]
- Xiang, H.F.; Li, Z.D.; Xie, K.; Jiang, J.Z.; Chen, J.J.; Lian, P.C.; Wu, J.S.; Wang, H.H. Graphene sheets as anode materials for Li-ion batteries: Preparation, structure, electrochemical properties and mechanism for lithium storage. RSC Adv. 2012, 2, 6792–6799. [Google Scholar] [CrossRef]
- Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Li storage properties of disordered graphene nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Yan, C.; Zhang, X.-Q.; Liu, H.; Zhang, Q. Electronic and Ionic Channels in Working Interfaces of Lithium Metal Anodes. ACS Energy Lett. 2018, 3, 1564–1570. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Npj Comput. Mater. 2018, 4, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.-B.; Zhao, C.-Z.; Yao, Y.-X.; Liu, H.; Zhang, Q. Recent Advances in Energy Chemistry between Solid-State Electrolyte and Safe Lithium-Metal Anodes. Chem 2019, 5, 74–96. [Google Scholar] [CrossRef] [Green Version]
- Single, B.H.F.; Latza, A. Revealing SEI Morphology: In-Depth Analysis of a Modeling Approach. J. Electrochem. Soc. 2017, 164, E3132–E3145. [Google Scholar] [CrossRef] [Green Version]
- Parr, R.G. Density Functional Theory. Annu. Rev. Phys. Chem. 1983, 34, 631–656. [Google Scholar] [CrossRef]
- Peter, M.W.; Gill, B.G.J. The performance of the Becke-Lee-Yang-Parr (B-LYP) density functional theory with various basis sets. Chem. Phys. Lett. 1992, 197, 499–505. [Google Scholar]
- Jeong, S.-K.; Inaba, M.; Iriyama, Y.; Abe, T.; Ogumi, Z. Surface film formation on a graphite negative electrode in lithium-ion batteries: AFM study on the effects of co-solvents in ethylene carbonate-based solutions. Electrochim. Acta 2002, 47, 1975–1982. [Google Scholar] [CrossRef]
- Borodin, O.; Ren, X.; Vatamanu, J.; von Wald Cresce, A.; Knap, J.; Xu, K. Modeling Insight into Battery Electrolyte Electrochemical Stability and Interfacial Structure. Acc. Chem. Res. 2017, 50, 2886–2894. [Google Scholar] [CrossRef]
- Gomes-Ballesteros, J.J.; Balbuena, P.B. Reduction of Electrolyte Components on a Coated Si Anode of Lithium-Ion Batteries. Phys. Chem. Lett. 2017, 8, 3404–3408. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]










| EC Ethylene Carbonate | DMC Dimethyl Carbonate | EMC Ethyl methyl Carbonate | DEC Diethyl Carbonate | |
|---|---|---|---|---|
| Molecular formula | C3H4O3 | C3H6O3 | C4H8O3 | C5H10O3 |
| Structural formula |  |  |  |  |
| Molecular weight | 88.06 | 90.08 | 104.11 | 118.13 |
| Density | 1.32 g/cm3 | 1.069 g/cm3 | 1.012 g/cm3 | 0.975 g/cm3 |
| Viscosity | 1.85 cP | 0.585 cP | 0.65 cP | 0.748 cP |
| Boiling point | 243 °C | 90.5 °C | 107.5 °C | 126 °C |
| Melting point | 38 °C | 3 °C | −14 °C | −43 °C |
| Component | System 1 | System 2 |
|---|---|---|
| GNS | 5 | 0 |
| HGNS | 0 | 5 |
| LiPF6 | 20 | 20 |
| EC | 360 | 360 |
| DMC | 48 | 48 |
| EMC | 40 | 40 |
| DEC | 132 | 132 |
| Sample | C (at.%) | O (at.%) | C/O (at.%) |
|---|---|---|---|
| GO | 62 | 38 | 1.6 |
| GNS | 98.3 | 1.7 | 58 |
| HGNS | 99.1 | 0.9 | 110 |
| Materials | Significant Findings Observed | Refs. |
|---|---|---|
| Holey graphene (HGNS); pristine graphene (GNS) | The HGNS anode exhibits a high specific capacity of 745 mAh/g at 0.1 A/g (after 80 cycles) and 141 mAh/g at a large current of 10 A/g (after 70 cycles), which are higher than the capacity values of the GNS counterpart by 75% and 130%, respectively. | This work |
| TiO2/GNS composite; GNS | The TiO2/GNS composite anode exhibits a specific capacity of ~170 mAh/g at 0.1 A/g (after 100 cycles) and ~70 mAh/g at a large current of 5 A/g (after 100 cycles). The GNS anode exhibits a specific capacity of ~78 mAh/g at 0.1 A/g (after 100 cycles) and ~20 mAh/g at a large current of 5 A/g (after 100 cycles). | [36] |
| GNS reduced from high-grade GO at 1050 °C; GNS reduced from medium-grade GO 1050 °C | The GNS reduced from high-grade GO has a specific surface area of 493 m2/g, and exhibits a reversible specific capacity of 835 mA/g at 0.05 A/g (1st cycle), which drops to ~700 mAh/g after 15 cycles. The GNS reduced from medium-grade GO has a specific surface area of 121m2/g, and exhibits a reversible specific capacity of 438 mAh/g at 0.05 A/g (1st cycle), which drops to ~320 mAh/g after 15 cycles. | [37] |
| Hydrazine reduced GNS; Electron-beam reduced GNS | The hydrazine reduced GNS anode exhibits a reversible capacity of ~330 mAh/g at 0.05 A/g (1st cycle). The e-beam reduce GNS anode exhibits reversible capacity of ~1054 mAh/g at 0.05 A/g (1st cycle), which drops to 784 mAh/g after 15 cycles. | [38] |
| GNS; GNS+CNT; GNS+C60 | The reversible capacity at a current density of 0.05 A/g is 540, 730, and 784 mAh/g for GNS, GNS+CNT, and GNS+C60, respectively. However, the reversible capacity after 20 cycles decays to 290,480, and 600 mAh/g for GNS, GNS+CNT, and GNS+ C60, respectively. | [3] |
| a-SnO2/graphene aerogel | The a-SnO2/graphene aerogel delivers a capacity of 700 mAh/g in 80th cycle at a current density of 0.1 A/g. At a larger current density of 1.6 A/g, it drops to 269 mAh/g (50th cycle). | [15] |
| AGN/S composite (3D porous network of activated graphene confining sulfur) | At rates of 0.5 C and 1 C, the composite electrode delivered high specific capacities of 1143 mAh/g and 927 mAh/g, respectively. After 100 cycles, the capacity decays to 766 and 686 mAh/g, respectively. | [19] |
| Li+ | EC | DMC | EMC | DEC | |
|---|---|---|---|---|---|
| GNS | 8.62 × 10−7 | 8.61 × 10−7 | 8.62 × 10−7 | 8.41 × 10−7 | 8.33 × 10−7 |
| HGNS | 9.98 × 10−7 | 9.96 × 10−7 | 9.93 × 10−7 | 9.96 × 10−7 | 9.96 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-R.; Chen, C.-L.; Pu, N.-W.; Wu, C.-H.; Liu, Y.-M.; Chen, Y.-H.; Youh, M.-J.; Ger, M.-D. Experimental and Modeling Analysis of Holey Graphene Electrodes for High-Power-Density Li-Ion Batteries. Crystals 2020, 10, 1063. https://doi.org/10.3390/cryst10111063
Huang Y-R, Chen C-L, Pu N-W, Wu C-H, Liu Y-M, Chen Y-H, Youh M-J, Ger M-D. Experimental and Modeling Analysis of Holey Graphene Electrodes for High-Power-Density Li-Ion Batteries. Crystals. 2020; 10(11):1063. https://doi.org/10.3390/cryst10111063
Chicago/Turabian StyleHuang, Yu-Ren, Cheng-Lung Chen, Nen-Wen Pu, Chia-Hung Wu, Yih-Ming Liu, Ying-Hsueh Chen, Meng-Jey Youh, and Ming-Der Ger. 2020. "Experimental and Modeling Analysis of Holey Graphene Electrodes for High-Power-Density Li-Ion Batteries" Crystals 10, no. 11: 1063. https://doi.org/10.3390/cryst10111063
APA StyleHuang, Y. -R., Chen, C. -L., Pu, N. -W., Wu, C. -H., Liu, Y. -M., Chen, Y. -H., Youh, M. -J., & Ger, M. -D. (2020). Experimental and Modeling Analysis of Holey Graphene Electrodes for High-Power-Density Li-Ion Batteries. Crystals, 10(11), 1063. https://doi.org/10.3390/cryst10111063