Ti3O5 and Al2TiO5 Crystals Flotation Characteristics from Ti-bearing Blast Furnace Slag: A Density Functional Theory and Experimental Study
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Analysis and Experiment Method
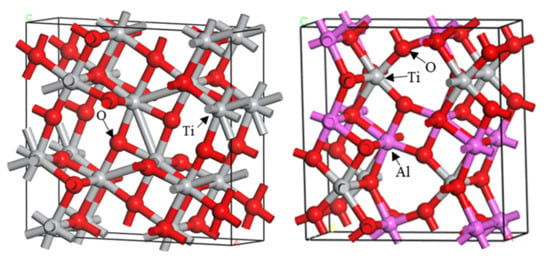
2.2.1. Construction Analysis of Ti3O5 and Al2TiO5 Crystalline Models
2.2.2. Flotation Experimental
3. Results and Discussion
3.1. Energy Bond Structures of Ti3O5 and Al2TiO5 Crystals
3.2. DOS Distributions of Ti3O5 and Al2TiO5 Crystals
3.3. Distributions of Ti3O5 and Al2TiO5 Atomic Charges
3.4. Flotability of Ti3O5 and Al2TiO5 Crystals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiong, Y.; Aldahri, T.; Liu, W.; Chu, G.; Zhang, G.; Luo, D.; Yue, H.; Liang, B.; Li, C. Simultaneous preparation of TiO2 and ammonium alum, and microporous SiO2 during the mineral carbonation of titanium-bearing blast furnace slag. Chin. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Hu, J.; Liu, Q.; Yue, H.; Liang, B.; Zhang, G.; Luo, D.; Xie, H.; Li, C. Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3. Chin. J. Chem. Eng. 2018, 26, 583–592. [Google Scholar] [CrossRef]
- Gao, J.; Li, C.; Liu, W.; Hu, J.; Wang, L.; Liu, Q.; Liang, B.; Yue, H.; Zhang, G.; Luo, D.; et al. Process simulation and energy integration in the mineral carbonation of blast furnace slag. Chin. J. Chem. Eng. 2019, 27, 157–167. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zhang, M.; Guo, M.; Wang, X. The Influence of SiO2 on the Extraction of Ti Element from Ti-bearing Blast Furnace Slag. Steel Res Int. 2011, 82, 607–614. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Wang, M.; Li, G.; Sui, Z. Dynamic oxidation of the Ti-bearing blast furnace slag. ISIJ Int. 2006, 46, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.H.; Lou, T.P.; Sui, Z.T. Study on Micrographics of Perovskite Crystallization. J. Northeastern Univ (Nat. Sci.). 2001, 22, 307–310. [Google Scholar]
- Guo, P.; Zhao, P. Technical analysis on selective separation and enrichment of Ti-bearing blast furance slag based on phase diagrams. Iron Steel Vanad. Titan. 2005, 26, 5–10. [Google Scholar]
- Xu, C.; Yuan, Z.; Wang, X. Preparation of TiCl4 with the Titanium Slag Containing Magnesia and Calcia in a Combined Fluidized Bed. Chin. J. Chem. Eng. 2006, 14, 281–288. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, J.L.; Wu, L.S.; Su, B.X.; Xing, X.D.; Zhu, G.Y. Effect of TiO2 on equilibrium phase sinter at oxygen partial pressure of 5 × 10−3 atm. Ironmak. Steelmak. 2014, 41, 132–137. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, J.L.; Xing, X.D.; Su, B.X.; Wang, Z.; Yan, B.J. Effect of B2O3 on phase compositions of high Ti bearing titanomagnetite sinter. Ironmak. Steelmak. 2013, 41, 500–506. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, J.; Wu, L.; Liu, W.; Bai, Y.; Xing, X.; Su, B.; Kong, D. Influence of B2O3 on Viscosity of High Ti-bearing Blast Furnace Slag. ISIJ Int. 2012, 52, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Liu, Q.C.; Zhang, J.L.; Chen, M.; Ma, X.; Zhao, B. Laboratory study of phase transitions and mechanism of reduction of FeO from high Ti-bearing blast furnace primary slag by graphite. Ironmak. Steelmak. 2014, 42, 117–125. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, J.L.; Liu, Q.C.; Chen, M.; Ma, X.; Li, K.J.; Zhao, B. Effect of B2O3 on reduction of FeO in Ti bearing blast furnace primary slag. Ironmak. Steelmak. 2014, 42, 498–503. [Google Scholar] [CrossRef]
- Ren, S.; Zhao, Q.; Yao, L.; Liu, Q. Precipitation behavior of perovskite and anosovite crystals from high Ti-bearing blast furnace slag with small amount of B2O3. CrystEngComm 2016, 18, 1393–1402. [Google Scholar] [CrossRef]
- Ren, S.; Guo, F.; Zhou, J.; Yang, J.; Yao, L.; Kong, M. Effect of compositions and additives content on crystallization behavior of Ti-rich phase from Ti-bearing blast furnace slag. Met. Res. Technol. 2017, 114, 415. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, J.L.; Liu, Q.C.; Li, K.J.; Zhao, B. Precipitation kinetics of anosovite in modified high Ti-bearing blast furnace slag. Metall. Res. Technol. 2015, 112, 105. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Wu, B.-Z.; Chen, J. Electronic structure and flotation behavior of monoclinic and hexagonal pyrrhotite. J. Central South Univ. 2015, 22, 466–471. [Google Scholar] [CrossRef]
- He, G.-C.; Xiang, H.-M.; Jiang, W.; Kang, Q.; Chen, J.-H. First-principles theory on electronic structure and floatability of spodumene. Rare Met. 2014, 33, 742–748. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wen, S.-M.; Feng, Q.-C.; Liu, J.; Ren, W.-C. Effects of magnesium and cooling rate on titanium phase transformation for production of TiO2. Trans. Nonferrous Met. Soc. China 2016, 26, 2518–2522. [Google Scholar] [CrossRef]
- Cohen, A.J.; Mori-Sánchez, P.; Yang, W. Insights into Current Limitations of Density Functional Theory. Sci. 2008, 321, 792–794. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, Y.-L.; Zhang, P.; He, C.; Deng, J.; Ding, S.; Shi, W. Combined DFT and XPS investigation of iodine anions adsorption on the sulfur terminated (001) chalcopyrite surface. Appl. Surf. Sci. 2016, 390, 412–421. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, Y.; Wen, S.; Deng, J.; Wu, D. The electronic structures of magnesium-bearing anosovite (MgnTi3-nO5 0 ≤ n ≤ 1) and its response to flotation. J. Alloy Compd. 2017, 708, 982–988. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Zeitschrift für Kristallographie - Crystalline Materials 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Dassault Systèmes BIOVIA. Materials Studio v2016; Dassault Systèmes: San Diego, CA, USA, 2016. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Mele, E.J.; Ritsko, J.J. Fermi-level lowering and the core exciton spectrum of intercalated graphite. Phys. Rev. Lett. 1979, 43, 68–71. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y. Summarization of relation between surface properties and Floatability of minerals. Gold J. 2000, 1, 30–33. [Google Scholar]
- Carta, M.; Ciccu, R.; Del Fa, C.; Ferrara, G.; Ghiani, M.; Massacci, P. The influence of the structure of mineral surface energy on electric separation and flotation. Proc. IX IMPC 1970, 4, 47–57. [Google Scholar]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO-MO molecular wave functions. III. effects of hybridization on overlap and gross AO populations. J. Chem. Phys. 1955, 23, 2338–2342. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. IV. Bonding and Antibonding in LCAO and Valence-Bond Theories. J. Chem. Phys. 1955, 23, 2343–2346. [Google Scholar] [CrossRef]
- Wang, X.-F.; Wang, Y.-J.; Wen, S.-M.; Yin, P.-G. Flotation behavior and adsorption mechanism of salicylhydroxamic acid in artificial mineral anosovite. J. Central South Univ. 2019, 26, 806–812. [Google Scholar] [CrossRef]







| Species | Atomic Populations (Mulliken) | Total/e | Charge/e | ||
|---|---|---|---|---|---|
| s | p | d | |||
| O | 1.84 | 4.83 | 0 | 6.67 | −0.67 |
| Ti | 2.26 | 6.31 | 2.30 | 10.87 | +1.12 |
| Species | Atomic Populations (Mulliken) | Total | Charge | ||
|---|---|---|---|---|---|
| s | p | d | |||
| O | 1.85 | 5.08 | 0 | 6.93 | −0.93 |
| Al | 0.51 | 0.87 | 0 | 1.38 | +1.63 |
| Ti | 2.22 | 6.22 | 2.17 | 10.61 | +1.39 |
| Bond | Population (e) | Length (Å) |
|---|---|---|
| Ti-O | 0.41 | 1.98 |
| Al-O | 0.46 | 1.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Su, Z.; Liu, W.; Sun, Y.; Li, X.; Yang, J. Ti3O5 and Al2TiO5 Crystals Flotation Characteristics from Ti-bearing Blast Furnace Slag: A Density Functional Theory and Experimental Study. Crystals 2020, 10, 838. https://doi.org/10.3390/cryst10090838
Ren S, Su Z, Liu W, Sun Y, Li X, Yang J. Ti3O5 and Al2TiO5 Crystals Flotation Characteristics from Ti-bearing Blast Furnace Slag: A Density Functional Theory and Experimental Study. Crystals. 2020; 10(9):838. https://doi.org/10.3390/cryst10090838
Chicago/Turabian StyleRen, Shan, Zenghui Su, Weizao Liu, Yali Sun, Xiaoming Li, and Jian Yang. 2020. "Ti3O5 and Al2TiO5 Crystals Flotation Characteristics from Ti-bearing Blast Furnace Slag: A Density Functional Theory and Experimental Study" Crystals 10, no. 9: 838. https://doi.org/10.3390/cryst10090838
APA StyleRen, S., Su, Z., Liu, W., Sun, Y., Li, X., & Yang, J. (2020). Ti3O5 and Al2TiO5 Crystals Flotation Characteristics from Ti-bearing Blast Furnace Slag: A Density Functional Theory and Experimental Study. Crystals, 10(9), 838. https://doi.org/10.3390/cryst10090838