Origins of a Low-Sulfur Superalloy Al2O3 Scale Adhesion Map
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cyclic Oxidation vs. Sulfur Database
3.2. Cyclic Oxidation Plots
3.3. Correlation with Sulfur Content
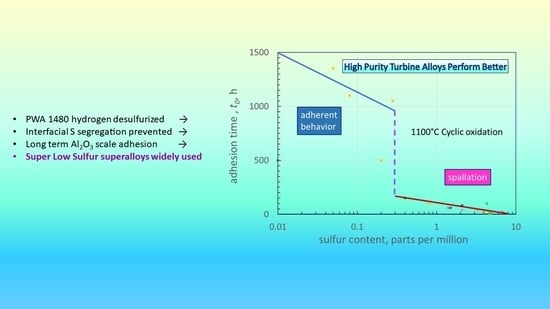
3.4. Scale-Adhesion Map
3.5. Segregation Projections
3.6. High Performing 2nd Generation Single Crystals
4. Summary and Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tubbs, B.K.; Smialek, J.L. Effect of Sulfur Removal on Scale Adhesion to PWA 1480. In Corrosion and Particle Erosion at High Temperatures; Srinivasan, V., Vedula, K., Eds.; TMS-AIME: Warrendale, PA, USA, 1989; pp. 459–487. [Google Scholar]
- Smialek, J.L.; Tubbs, B.K. Effect of sulfur removal on scale adhesion to PWA 1480. Metall. Mat. Trans. A 1995, 26, 427–435. [Google Scholar] [CrossRef]
- Smialek, J.L. Oxidation Resistance and Critical Sulfur Content of Single Crystal Superalloys. J. Engineer. Gas Turb. Power, ASME Trans. 1998, 120, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Smialek, J.L. Effect of Hydrogen Annealing and Sulfur Content on the Oxidation Resistance of PWA 1480. PPM. Other Propuls. R T 1997, 1, 1–13. [Google Scholar]
- Smialek, J.L. Maintaining Adhesion of Protective Al2O3 Scales. JOM 2000, 52, 22–26. [Google Scholar] [CrossRef]
- Smialek, J.L. Toward Optimum Scale and TBC Adhesion on Single Crystal Superalloys. In High Temperature Corrosion and Materials Chemistry; Opila, E.J., Hou, P.Y., Shores, D., McNallan, M., Oltra, R., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1998; Volume 98–99, pp. 211–220. [Google Scholar]
- Smialek, J.L.; Auping, J.V. COSP for Windows: Strategies for Rapid Analyses of Cyclic Oxidation Behavior. Oxid. Met. 2002, 57, 559–581. [Google Scholar] [CrossRef]
- Jayne, D.T.; Smialek, J.L. A Sulfur Segregation study of PWA 1480, NiCrAl, and NiAl Alloys. In Microscopy of Oxidation II; Newcomb, S.B., Bennett, M.J., Eds.; Institute of Metals: London, UK, 1993; pp. 183–196. [Google Scholar]
- Hou, P.Y. Segregation Phenomena at Thermally Grown Al2O3/Alloy Interfaces. Annu. Rev. Mater. Res. 2008, 38, 275–298. [Google Scholar] [CrossRef]
- Miyahara, T.; Stolt, K.; Reed, D.A.; Birnbaum, H.K. Sulfur Segregation on Nickel. Scripta Met. 1985, 19, 117–121. [Google Scholar] [CrossRef]
- Smialek, J.L.; Pint, B.A. Optimizing Scale Adhesion for Single Crystal Superalloys. Mater. Sci. Forum 2001, 369, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Smialek, J.L. Scale Adhesion, Sulfur Content, and TBC Failure on Single Crystal Superalloys. In Proceedings of the 26th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: B: Ceramic En-gineering and Science Proceedings, Cocoa Beach, FL, USA, 1 January 2002; pp. 485–495. [Google Scholar]
- Smialek, J.L. Improved Oxidation Life of Segmented Plasma Sprayed 8YSZ Thermal Barrier Coatings. J. Therm. Spray Technol. 2004, 13, 66–75. [Google Scholar] [CrossRef]
- Smith, M.A.; Pregger, B.A. Effect of sulfur on the cyclic oxidation behavior of a single crystalline, nickel-base superalloy. Mat. Sci. Eng. A 1995, 203, 388–398. [Google Scholar] [CrossRef]
- Irvine, J.D.; Vogt, R.G.; Bierstine, D.L.; Stabile, C.M.; Mihalisin, J.R.; Smith, J.S.; Kunkle, J.P.; Cole, G.R.; Nielsen, T.W. Ultra Low Sulfur Superalloy. US Patent 5,922,148, 13 July 1999. [Google Scholar]
- Gray, S.; Harris, K. Cranfield and Cannon-Muskegon. Unpublished work. 2017. [Google Scholar]
- Harris, K.; Cannon-Muskegon. Personal communication, 2020.





| Sample # | 10-7 | 10-6 | 10-1 | 10-2 | 10-4 |
|---|---|---|---|---|---|
| L, mm | 0.18 | 0.18 | 0.12 | 0.14 | 0.18 |
| H2, (°C/h.) | 1000/8 | 1000/20 | 1000/100 | 1200/20 | 1200/100 |
| h.\CS (ppmw) | 4.9 | 4.6 | 4.3 | 0.15 | 0.07 |
| 0 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.25 | 0.23 | 0.20 | 0.21 | 0.23 |
| 5 | 0.03 | 0.26 | 0.23 | 0.26 | 0.27 |
| 10 | 0.07 | 0.00 | 0.25 | 0.29 | 0.29 |
| 20 | −0.21 | −0.16 | 0.27 | 0.33 | 0.33 |
| 40 | −1.32 | −1.24 | 0.27 | 0.39 | 0.38 |
| 60 | −2.14 | −1.92 | 0.25 | 0.42 | 0.42 |
| 80 | −2.98 | −2.58 | 0.15 | 0.42 | 0.42 |
| 100 | −3.77 | −3.21 | 0.03 | 0.46 | 0.46 |
| 150 | −5.48 | −4.86 | −0.27 | 0.52 | 0.52 |
| 200 | −6.95 | −5.98 | −0.70 | 0.56 | 0.57 |
| 250 | −9.24 | −7.41 | −1.06 | 0.58 | 0.60 |
| 300 | −10.43 | −8.78 | −1.49 | 0.61 | 0.63 |
| 400 | −13.48 | −10.49 | −2.35 | 0.60 | 0.65 |
| 500 | −24.33 | −18.35 | −2.57 | 0.58 | 0.65 |
| Sample # | 20-0 | 20-1 | 20-8 | 20-5 | 20-3 | 20-4 | 20-9 | 20-6 | 20-7a | 20-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| L. mm | 0.42 | 0.45 | 0.44 | 0.43 | 0.41 | 0.39 | 0.39 | 0.42 | 0.42 | 0.40 |
| H2, (°C/h.) | none | 1000/20 | 1100/20 | 1200/8 | 1200/20 | 1200/20 | 1200/50 | 1200/100 | 1200/100 | 1300/20 |
| h.\CS (ppmw) | 6.7 | 3.9 | 2.1 | 0.8 | 0.28 | N.A. | 0.05 | 0.08 | 0.2 | 0.06 |
| 0 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.23 | 0.23 | 0.24 | 0.23 | 0.23 | 0.22 | 0.21 | 0.21 | 0.16 | 0.23 |
| 5 | 0.28 | 0.30 | 0.27 | 0.27 | 0.26 | 0.25 | 0.25 | 0.25 | 0.21 | 0.25 |
| 10 | 0.27 | 0.26 | 0.32 | 0.29 | 0.31 | 0.30 | 0.29 | 0.27 | 0.24 | 0.28 |
| 20 | −0.16 | 0.15 | 0.32 | 0.33 | 0.32 | 0.31 | 0.33 | 0.30 | 0.30 | 0.32 |
| 40 | −1.18 | −0.65 | 0.01 | 0.36 | 0.36 | 0.35 | 0.39 | 0.34 | 0.37 | 0.34 |
| 40 | −1.25 | −0.72 | −0.07 | 0.32 | 0.37 | 0.35 | 0.39 | 0.34 | 0.35 | 0.33 |
| 60 | −2.73 | −1.85 | −1.05 | 0.10 | 0.52 | 0.50 | 0.52 | 0.44 | 0.49 | 0.34 |
| 80 | −3.24 | −2.27 | −1.92 | 0.07 | 0.53 | 0.53 | 0.54 | 0.46 | 0.52 | 0.31 |
| 100 | −3.98 | −3.25 | −2.97 | −0.01 | 0.55 | 0.54 | 0.55 | 0.47 | 0.53 | 0.29 |
| 150 | −5.64 | −4.83 | −4.70 | −0.16 | 0.57 | 0.58 | 0.60 | 0.51 | 0.57 | 0.24 |
| 200 | −7.44 | −6.07 | −6.27 | −0.34 | 0.62 | 0.62 | 0.65 | 0.54 | 0.61 | 0.16 |
| 200 | −8.01 | −6.42 | −6.61 | −0.48 | 0.57 | 0.57 | 0.60 | 0.49 | 0.60 | 0.03 |
| 250 | −12.08 | −9.13 | −9.46 | −0.81 | 0.58 | 0.59 | 0.60 | 0.50 | 0.59 | −0.05 |
| 300 | −15.32 | −12.61 | −12.85 | −1.11 | 0.62 | 0.62 | 0.63 | 0.52 | 0.61 | −0.14 |
| 400 | −23.89 | −18.18 | −18.57 | −1.79 | 0.63 | 0.65 | 0.63 | 0.49 | 0.65 | −0.42 |
| 500 | −32.55 | −24.81 | −25.85 | −2.61 | 0.59 | 0.64 | 0.65 | 0.49 | 0.68 | −0.77 |
| 500 | −32.86 | −24.99 | −26.05 | −2.67 | 0.58 | 0.62 | 0.67 | 0.46 | 0.65 | −0.83 |
| 600 | −3.28 | 0.52 | 0.57 | 0.39 | −1.13 | |||||
| 700 | −3.77 | 0.49 | 0.54 | 0.37 | −1.41 | |||||
| 800 | −4.37 | 0.45 | 0.50 | 0.35 | −1.68 | |||||
| 900 | −5.22 | 0.31 | 0.46 | 0.31 | −2.24 | |||||
| 1000 | −6.74 | 0.10 | 0.36 | 0.16 | −3.38 |
| Sample # | 50-0 | 50-4 | 50-3 | 50-5 | 50-2 |
|---|---|---|---|---|---|
| L, mm | 1.20 | 1.19 | 1.18 | 1.24 | 1.27 |
| H2, (°C/h.) | control | 1200/8 | 1200/20 | 1200/100 | 1300/20 |
| h.\CS (ppmw) | N.A. | 2.0 | 1.5 | 0.12 | 0.34 |
| 0 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.20 | 0.22 | 0.24 | 0.22 | 0.04 |
| 5 | 0.30 | 0.26 | 0.29 | 0.26 | 0.07 |
| 10 | 0.25 | 0.20 | 0.33 | 0.30 | 0.10 |
| 20 | −0.05 | 0.10 | 0.33 | 0.38 | 0.10 |
| 40 | −0.98 | −0.25 | 0.23 | 0.46 | 0.02 |
| 60 | −1.95 | −0.97 | 0.04 | 0.55 | −0.14 |
| 80 | −2.82 | −1.81 | −0.25 | 0.59 | −0.29 |
| 100 | −3.70 | −2.65 | −0.65 | 0.65 | −1.06 |
| 150 | −5.93 | −4.71 | −2.05 | 0.79 | −2.03 |
| 200 | −8.08 | −6.78 | −3.66 | 0.73 | −3.08 |
| 250 | −10.80 | −8.92 | −5.08 | 0.77 | −4.17 |
| 300 | −16.95 | −11.93 | −6.48 | 0.76 | −6.39 |
| 400 | −33.94 | −23.21 | −9.35 | 0.72 | −6.39 |
| 500 | −66.85 | −37.90 | −12.51 | 0.75 | −8.68 |
| Sample # | 100-2 | 100-1 | 100-3 | 100-4 | 200-1 | 200-2 |
|---|---|---|---|---|---|---|
| L, mm | 2.54 | 2.53 | 2.51 | 2.59 | 5.06 | 5.12 |
| H2, (°C/h.) | 1100/100 | 1200/20 | 1200/100 | 1300/100 | 1200/100 | 1300/20 |
| h.\CS (ppmw) | 2.1 | 1.5 | 0.4 | 0.01 | 1.4 | 3.3 |
| 0 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.31 | 0.29 | 0.33 | 0.30 | 0.24 | 0.25 |
| 5 | 0.35 | 0.33 | 0.38 | 0.36 | 0.28 | 0.34 |
| 10 | 0.39 | 0.36 | 0.44 | 0.40 | 0.31 | 0.34 |
| 20 | 0.43 | 0.33 | 0.49 | 0.43 | 0.26 | 0.23 |
| 40 | 0.38 | 0.17 | 0.53 | 0.50 | 0.15 | −0.19 |
| 60 | 0.17 | −0.01 | 0.53 | 0.53 | −0.03 | −0.79 |
| 80 | −0.11 | −0.36 | 0.53 | 0.55 | −0.25 | −1.45 |
| 100 | −0.60 | −0.98 | 0.45 | 0.55 | −0.53 | −2.22 |
| 150 | −2.62 | −2.79 | 0.10 | 0.53 | −1.44 | −3.88 |
| 200 | −4.66 | −4.41 | −0.49 | 0.47 | −2.75 | −5.56 |
| 300 | −8.90 | −7.86 | −2.07 | 0.34 | −5.60 | −9.29 |
| 400 | −15.35 | −11.97 | −3.75 | 0.21 | −8.34 | −14.57 |
| 500 | −26.34 | −20.55 | −5.67 | 0.06 | −12.82 | −27.52 |
| H2: Temp. | 1000 °C | 1100 °C | 1200 °C | 1300 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cs | DW/A500 | t0 | Cs | DW/A500 | t0 | Cs | DW/A500 | t0 | Cs | DW/A500 | t0 | |
| H2: Time | ppmw | mg/cm2 | h | ppmw | mg/cm2 | h | ppmw | mg/cm2 | h | ppmw | mg/cm2 | h |
| L = 0.15 mm | ||||||||||||
| 8 | 4.9 | −24.33 | 10 | |||||||||
| 20 | 4.6 | −18.36 | 10 | 0.15 | 0.58 | >500 | ||||||
| 100 | 4.3 | −2.57 | 100 | 0.07 | 0.65 | >500 | ||||||
| L = 0.42 mm | ||||||||||||
| 0 | 6.7 | −32.86 | 20 | |||||||||
| 8 | 0.8 | −2.62 | 100 | |||||||||
| 20 | 3.9 | −24.99 | 20 | 2.1 | −26.05 | 40 | 0.28 | 0.59 | 1050 | 0.06 | −0.83 | 40 |
| 20 | N.A. | 0.64 | >500 | |||||||||
| 50 | 0.05 | 0.65 | 1350 | |||||||||
| 100 | 0.08 | 0.68 | 1100 | |||||||||
| 100 | 0.2 | 0.49 | >500 | |||||||||
| L = 1.22 mm | ||||||||||||
| 8 | 2 | −37.90 | 20 | |||||||||
| 20 | 1.5 | −12.52 | 60 | 0.34 | −8.68 | 40 | ||||||
| 100 | 0.12 | 0.75 | >500 | |||||||||
| L = 2.53 mm | ||||||||||||
| 20 | 1.5 | −20.55 | 60 | |||||||||
| 100 | 2.1 | −26.34 | 80 | 0.4 | −5.67 | 150 | 0.01 | 0.06 | 500 | |||
| L = 5.09 mm | ||||||||||||
| 20 | 3.3 | −27.52 | 40 | |||||||||
| 100 | 1.4 | −12.82 | 60 | |||||||||
| Alloy | T (°C) | t (h) | ppmw S | W/A (mg/cm2) | Process | Study |
|---|---|---|---|---|---|---|
| PWA 1484 | 1100 | 2000 | 0.01 | 0.93 | H | a, b |
| PWA 1484 | 1100 | 2000 | 0.25 | 0.77 | M | a, b |
| PWA 1484 | 1150 | 1000 | 0.01 | 0.10 | H | a, b |
| PWA 1484 | 1150 | 1000 | 0.25 | 0.52 | M | a, b |
| PWA 1484 | 1177 | 600 | 0.2 | −0.56 | M | c |
| Rene’N5 | 1150 | 1000 | 0.01 | 0.80 | H | d |
| Rene’N6 | 1200 | 200 | 0.08 | −0.80 | H | e |
| CMSX-4®(SLS) | 1135 | 680 | 0.25 | 0.48 | M | f |
| CMSX-4®(SLS) | 1200 | 500 | 0.25 | −4.90 | M | f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smialek, J. Origins of a Low-Sulfur Superalloy Al2O3 Scale Adhesion Map. Crystals 2021, 11, 60. https://doi.org/10.3390/cryst11010060
Smialek J. Origins of a Low-Sulfur Superalloy Al2O3 Scale Adhesion Map. Crystals. 2021; 11(1):60. https://doi.org/10.3390/cryst11010060
Chicago/Turabian StyleSmialek, James. 2021. "Origins of a Low-Sulfur Superalloy Al2O3 Scale Adhesion Map" Crystals 11, no. 1: 60. https://doi.org/10.3390/cryst11010060
APA StyleSmialek, J. (2021). Origins of a Low-Sulfur Superalloy Al2O3 Scale Adhesion Map. Crystals, 11(1), 60. https://doi.org/10.3390/cryst11010060