Structure and Properties of Ln2MoO6 Oxymolybdates (Ln = La, Pr, Nd) Doped with Magnesium
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
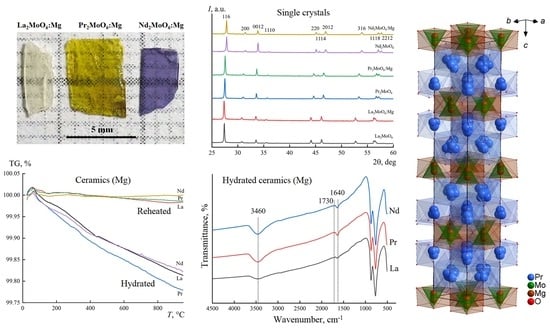
3.1. Powder X-ray Diffraction
3.2. Thermogravimetry
3.3. IR Spectroscopy
3.4. Crystal Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blasse, G. Dilanthanide molybdate and tungstates Ln2MoO6. J. Inorg. Nucl. Chem. 1966, 28, 1488–1489. [Google Scholar] [CrossRef]
- Pokrovskii, А.N.; Rybakov, V.K.; Trunov, V.K. XRD study of oxytungstate and oxymolybdate of lanthanides and ittrium oxymolybdate. Zhurnal Neorg. Khimii 1969, 14, 2344–2347. [Google Scholar]
- McCarthy, G.J.; Fischer, R.D.; Johnson, G.G., Jr.; Gooden, C.E. Crystal Chemistry and Compound Formation in the Systems Rare Earth Sesquioxide—WO3. In Proceedings of the Fifth Material Research Symposium on Solid State Chemistry, Gaithersburg, MD, USA, 18 October 1971; pp. 397–411. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:4068137 (accessed on 27 May 2021).
- Brixner, L.N.; Sleight, A.W.; Licis, M.S. La2MoO6—Type rare earth molybdates—Preparation and lattice parameters. J. Solid State Chem. 1972, 5, 186–190. [Google Scholar] [CrossRef]
- Dorn, K.V.; Hartenbach, I. Press to success: Gd5FW3O16—The first gadolinium(III) fluoride oxidotungstate(VI). Crystals 2019, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Errandonea, D.; Ruiz-Fuertes, J. A brief review of the effects of pressure on wolframite-type oxides. Crystals 2018, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Nassau, K.; Levinstein, H.J.; Loiacono, G.M. A comprehensive study of trivalent tungstates and molybdates of the type L2(MO4)3. J. Phys. Chem. Solids 1965, 26, 1805–1816. [Google Scholar] [CrossRef]
- Lacorre, P.; Goutenoire, F.; Bohnke, O.; Retoux, R.; Laligant, Y. Designing fast oxide-ion conductors based on La2Mo2O9. Nat. Cell Biol. 2000, 404, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.H.; Michel, P.; Thozet, A. Structure of neodymium molybdenite Nd5Mo3O16. C. R. Hebd. Seances Acad. Sci. Ser. C 1973, 276, 1779–1781. [Google Scholar]
- Meng, F.; Zhang, X.; Li, H.; Seo, H.J. Synthesis and spectral characteristics of La2MoO6:Ln3+ (Ln = Eu, Sm, Dy, Pr, Tb) polycrystals. J. Rare Earths 2012, 30, 866–870. [Google Scholar] [CrossRef]
- Colmont, M.; Boutinaud, P.; Latouche, C.; Massuyeau, F.; Huvé, M.; Zadoya, A.; Jobic, S. Origin of luminescence in La2MoO6 and La2Mo2O9 and their Bi-doped variants. Inorg. Chem. 2020, 59, 3215–3220. [Google Scholar] [CrossRef]
- Chen, Y.C.; Weng, M.Z. Improving quality factor of Nd2MoO6 ceramics by removing moisture content. J. Mater. Sci. Mater. Electron. 2015, 26, 3502–3505. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, H.; Zhou, H.; Luan, X.; Wang, K.; Chen, X. Phase structure, sintering behaviour and microwave dielectric properties of Ln2MoO6 (Ln = La and Y) ceramics. Ceram. Int. 2020, 46, 24552–24556. [Google Scholar] [CrossRef]
- Sillen, L.G.; Lundburg, K. La2MoO6, ein Lanthanoxymolybdat mit Schichtenstructur. Z. Anorg. Chem. 1943, 252, 2–8. [Google Scholar] [CrossRef]
- Klevtsov, P.V.; Khartchenko, L.Y.; Klevtsova, R.F. Crystallization and polymorphism of rare-earth oxymolybdates of composition Ln2MoO6. Kristallografiya 1975, 20, 571–578. [Google Scholar]
- Efremov, V.A.; Tyulin, A.V.; Trunov, V.K. Actual structure of tetragonal Ln2O2MoO4 and factors, determining the distortion of structure—Forming coordination polyhedral. Koord. Khim. 1987, 13, 1276–1282. [Google Scholar]
- Xue, J.S.; Antonio, M.R.; Soderholm, L. Polymorphs of Ln2MoO6: A neutron diffraction of the crystal structures of La2MoO6 and Tb2MoO6. Chem. Mater. 1995, 7, 333–340. [Google Scholar] [CrossRef]
- Polyanskaya, T.M.; Borisov, S.V.; Belov, N.V. A new form of the sheelite structural type: Crystal structure of Nd2WO6. Dokl. Akad. Nauk SSSR 1970, 193, 83–86. [Google Scholar]
- Evdokimov, A.A.; Efremov, V.A.; Trunov, V.K.; Kleinman, I.A.; Dzhurinskyi, B.Ph. Rare-Earth Compounds. Molybdates and Tungstates; Nauka: Moscow, Russia, 1991. [Google Scholar]
- Antipin, A.M.; Sorokina, N.I.; Alekseeva, O.A.; Dudka, A.P.; Chernyshev, D.Y.; Voronkova, V.I. Polymorphism and structure of Nd2MoO6 single crystals. Crystallogr. Rep. 2017, 62, 537–544. [Google Scholar] [CrossRef]
- Voronkova, V.; Orlova, E.; Kazakov, S.; Kharitonova, E.; Belov, D. Phase relations and physical properties of layered Pb-containing Nd2MoO6 compounds. Eur. J. Inorg. Chem. 2016, 2016, 1022–1029. [Google Scholar] [CrossRef]
- Kuzmicheva, G.M. Main Crystal Chemical Categories; MITHT: Moscow, Russia, 2001. [Google Scholar]
- Yanovskii, V.K.; Voronkova, V.I. Electroconductivity of tungstates and molybdates of A2BO6 and Meyer–Neldel rule. Solid State Physics. 1977, 19, 3318–3321. [Google Scholar]
- Voronkova, V.I.; Kharitonova, E.P.; Orlova, E.I.; Sorokina, N.I.; Sorokin, T.A.; Antipin, A.M.; Baldin, E.D.; Grebenev, V.V. Synthesis, structure, and physical properties of layered tetragonal Mg-doped Nd2MoO6 compounds. J. Alloy. Compd. 2019, 803, 1045–1053. [Google Scholar] [CrossRef]
- Voronkova, V.I.; Antipin, A.M.; Sorokin, T.A.; Novikova, N.E.; Kharitonova, E.P.; Orlova, E.I.; Kvartalov, V.B.; Presniakov, M.Y.; Bondarenko, V.I.; Vasiliev, A.L.; et al. Synthesis, structure and properties of layered Pr2MoO6-based oxymolybdates doped with Mg. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 492–501. [Google Scholar] [CrossRef]
- Orlova, E.I.; Kharitonova, E.P.; Sorokina, N.I.; Sorokin, T.A.; Antipin, A.M.; Voronkova, V.I. Structure and physical properties of Mg-containing oxymolybdates La2MoO6. Crystallogr. Rep. 2020, 65, 697–703. [Google Scholar] [CrossRef]
- Voronkova, V.; Kharitonova, E.; Orlova, E.; Gorshkov, N.; Goffman, V. Synthesis and unusual properties of tetragonal Pb-containing oxymolybdates based on La2MoO6. Eur. J. Inorg. Chem. 2017, 2017, 5582–5587. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.39.46; Rigaku Corporation: Oxford, UK, 2018. [Google Scholar]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Palatinus, L. Ab initio determination of incommensurately modulated structures by charge flipping in superspace. Acta Crystallogr. 2004, 60, 604–610. [Google Scholar] [CrossRef]
- Colomban, P. Proton and protonic species: The hidden face of solid state chemistry. How to measure H-content in materials? Fuel Cells 2012, 13, 6–18. [Google Scholar] [CrossRef]
- Fomichev, V.V.; Kondratov, O.I.; Gagarin, V.A.; Gokhman, L.Z.; Petrov, K.I. Investigation of vibrational spectra of oxymolybdates of rare earth elements. Zhurnal Neorganicheskoj Khimii 1977, 22, 2150–2157. [Google Scholar]
- Yukhnevich, G.V. Infrared Spectroscopy of Water; Nauka: Moscow, Russia, 1973. [Google Scholar]
- Animitsa, I.E. Proton Transport. In Complex. Oxides. Tutorial; Ural University Publishing House: Ekaterinburg, Russia, 2014. [Google Scholar]










| Composition | a, c, Å | V, Å3 |
|---|---|---|
| (MgO)x(La2MoO6)(1–x)/2, x = 0 | 5.795(7), 32.054(4) | 1076.44(5) |
| (MgO)x(La2MoO6)(1–x)/2, x = 0.03 | 5.791(2), 32.03(1) | 1074.15(2) |
| (MgO)x(Pr2MoO6)(1–x)/2, x = 0 | 5.693(9), 31.656(4) | 1039.7(9) |
| (MgO)x(Pr2MoO6)(1–x)/2, x = 0.03 | 5.691(1), 31.646(5) | 1040.68(0) |
| (MgO)x(Nd2MoO6)(1–x)/2, x = 0 | 5.665(1), 31.638(5) | 1015.33(4) |
| (MgO)x(Nd2MoO6)(1–x)/2, x = 0.03 | 5.659(1), 31.622(9) | 1012.8(5) |
| (MgO)x(Nd2MoO6)(1–x)/2, x = 0.05 | 5.662(1), 31.616(8) | 1013.5(4) |
| Atom | Ln | I41/acd | Δ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x/a | y/b | z/c | x/a | y/b | z/c | x/a | y/b | z/c | ||
| La1 | La | 0 | 0 | 0.16351(1) | 0 | 0 | 0.16343(1) | 0 | 0 | 0.00008 |
| La1i/La2 | 0.5 | 0 | 0.08649(1) | 0.5 | 0 | 0.08639(1) | 0 | 0 | 0.0001 | |
| Mo1 | 0.5 | 0 | 0.25 | 0.5 | 0 | 0.25 | 0 | 0 | 0 | |
| Mo1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pr1 | Pr | 0 | 0 | 0.16311(1) | 0 | 0 | 0.16318(1) | 0 | 0 | –0.00007 |
| Pr1i/Pr2 | 0.5 | 0 | 0.08689(1) | 0.5 | 0 | 0.08698(1) | 0 | 0 | –0.00009 | |
| Mo1 | 0.5 | 0 | 0.25 | 0.5 | 0 | 0.25 | 0 | 0 | 0 | |
| Mo1i/Mo2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nd1 | Nd | 0 | 0 | 0.16294(1) | 0 | 0 | 0.16323(1) | 0 | 0 | –0.00029 |
| Nd1i/Nd2 | 0.5 | 0 | 0.08706(1) | 0.5 | 0 | 0.08734(1) | 0 | 0 | –0.00028 | |
| Mo1 | 0.5 | 0 | 0.25 | 0.5 | 0 | 0.25 | 0 | 0 | 0 | |
| Mo1i/Mo2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| O1 | La | 0.75 | 0.2409(2) | 0.125 | 0.7443(1) | 0.2411(1) | 0.1249(1) | 0.0057 | –0.0002 | 0.0001 |
| O2_1 | 0.3329(1) | 0.1683(1) | 0.2147(1) | 0.3341(1) | 0.1683(1) | 0.2148(1) | –0.0012 | 0 | –0.0001 | |
| O2_1ii/O2_2 | 0.6683(1) | 0.3329(1) | 0.4647(1) | 0.6682(1) | 0.3312(1) | 0.5353(1) | 0.0001 | 0.017 | –0.0706 | |
| O1 | Pr | 0.75 | 0.2669(3) | 0.125 | 0.7322(1) | 0.2459(6) | 0.1255(1) | 0.0178 | 0.021 | –0.0005 |
| O2_1 | 0.3355(1) | 0.1758(1) | 0.2143(1) | 0.3322(1) | 0.1691(3) | 0.2871(1) | 0.0033 | 0.0067 | –0.0728 | |
| O2_1ii/O2_2 | 0.6758(1) | 0.3354(1) | 0.4643(1) | 0.6733(1) | 0.3295(4) | 0.4662(1) | 0.0025 | 0.0059 | –0.0019 | |
| O1 | Nd | 0.75 | 0.2567(1) | 0.125 | 0.7524(1) | 0.2567(1) | 0.1249(1) | –0.0024 | 0 | 0.0001 |
| O2_1 | 0.3348(1) | 0.1757(1) | 0.2859(1) | 0.3314(1) | 0.1679(1) | 0.2859(1) | 0.0034 | 0.0078 | 0 | |
| O2_1ii/O2_2 | 0.6757(1) | 0.1757(1) | 0.5359(1) | 0.6793(1) | 0.3337(1) | 0.5359(1) | –0.0036 | 0.0011 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, E.; Kharitonova, E.; Sorokin, T.; Antipin, A.; Novikova, N.; Sorokina, N.; Voronkova, V. Structure and Properties of Ln2MoO6 Oxymolybdates (Ln = La, Pr, Nd) Doped with Magnesium. Crystals 2021, 11, 611. https://doi.org/10.3390/cryst11060611
Orlova E, Kharitonova E, Sorokin T, Antipin A, Novikova N, Sorokina N, Voronkova V. Structure and Properties of Ln2MoO6 Oxymolybdates (Ln = La, Pr, Nd) Doped with Magnesium. Crystals. 2021; 11(6):611. https://doi.org/10.3390/cryst11060611
Chicago/Turabian StyleOrlova, Ekaterina, Elena Kharitonova, Timofei Sorokin, Alexander Antipin, Nataliya Novikova, Nataliya Sorokina, and Valentina Voronkova. 2021. "Structure and Properties of Ln2MoO6 Oxymolybdates (Ln = La, Pr, Nd) Doped with Magnesium" Crystals 11, no. 6: 611. https://doi.org/10.3390/cryst11060611
APA StyleOrlova, E., Kharitonova, E., Sorokin, T., Antipin, A., Novikova, N., Sorokina, N., & Voronkova, V. (2021). Structure and Properties of Ln2MoO6 Oxymolybdates (Ln = La, Pr, Nd) Doped with Magnesium. Crystals, 11(6), 611. https://doi.org/10.3390/cryst11060611