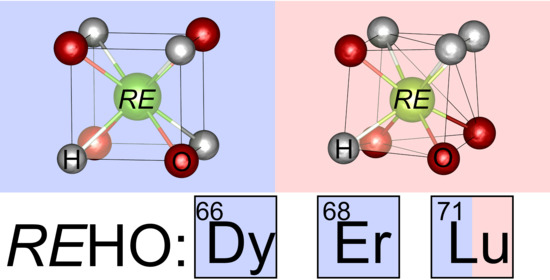
1. Introduction
The hydride ion H
− has a unique chemical character due to its medium electronegativity, high polarizability, and small size, and is thus a promising candidate in the design of functional materials. As stable ionic hydrides are only known for compounds of lowly electronegative cations, e.g., alkaline and alkaline earth elements, the chemical space of binary ionic hydrides is rather limited. In recent years however, a rising number of so-called heteroanionic or mixed anionic hydrides is being investigated that contain additional anions and thus expand the accessible chemical space of ionic hydrides [
1,
2,
3,
4]. These compounds show several interesting properties such as hydride ion conductivity [
5,
6,
7,
8,
9,
10,
11], catalytic activity [
12,
13,
14,
15,
16], superconductivity [
17,
18], and luminescence [
19,
20,
21,
22,
23,
24,
25] or photochromism [
26,
27,
28,
29,
30].
Ternary rare earth hydride oxides (or oxyhydrides) LaH
xO (
x = 0.78), CeH
xO (
x = 0.90), PrH
xO (
x = 0.56),
REH
1+2xO
1−x (
RE: rare earth element;
RE = La, Ce, Pr; 0 <
x ≤ 0.2), and LaH
1+xO
1−y (0 <
y ≤ 0.2,
x < 2
y) were first reported in the 1960s and 1980s [
31,
32]. In all cases, the CaF
2 (fluorite) structure type with space group
Fm3m was assigned, similar to the cubic rare earth oxide fluorides [
33]. The crystal structure can be described as a cubic close packing of rare earth atoms whose tetrahedral interstices are occupied by hydrogen and oxygen in a disordered fashion. Recently, the structure of LaH
1+2xO
1−x and its compositional range with respect to H and O were further investigated, and a tetragonal superstructure was assigned. It shows a broad phase width with increasing occupation of the octahedral interstice by hydrogen [
6,
34], similar to LaH
2+x [
35], and full mixing of anions. The 1:1:1 compounds
REHO (sometimes called “stoichiometric” hydride oxides,
RE = La, Ce, Pr, Nd) form a CaF
2 superstructure with tetragonal symmetry and space group
P4/
nmm, which was later confirmed by neutron diffraction analysis on LaHO and NdHO (
Figure 1, bottom center) [
7,
31,
36,
37]. A recent study furthermore observed a monoclinically distorted modification with space group
P12
1/
m1 [
34]. At high pressures, the tetragonal LaHO structure transforms to the hexagonal anti-Fe
2P type via the orthorhombic PbCl
2 type structure [
34,
38]. Upon heating, tetragonal LaHO showed a reduction in the intensities of the fluorite superstructure reflections similar to LaOF [
39], which is why a fluorite type high-temperature modification was assumed [
31]. For the lanthanum compounds, the composition had a great influence on the transport properties: while LaHO showed low and LaH
1+2xO
1−x high ionic conductivity depending on
x, LaH
1+xO
1−y was metallically conducting [
6,
31].
The smaller rare earth elements crystallize in the cubic fluorite structure type with disordered anions (
Fm3m,
RE = Y, Sm, Gd–Er; confirmed by neutron powder diffraction analysis on YHO, HoHO, ErHO) [
7,
12,
20,
40,
41] or ordered varieties thereof, the so-called half-Heusler LiAlSi structure type with space group
F43
m (
RE = Ho) [
42] and the orthorhombically distorted anti-LiMgN structure type with space group
Pnma (
RE = Y; also observed in monoclinic distortion with space group
P12
1/
m1 similar to the lanthanum compound (see above);
Figure 1) [
34,
41]. The phase width of these compounds was investigated on the yttrium system Y–H–O [
34]. It is significantly smaller than the analogous lanthanum system due to the less efficient shielding of anionic charges during occupation of the octahedral interstices. The reported compounds YH
1+2xO
1−x are isotypic to the lanthanum representatives above. Oxygen-rich phases, e.g.,
REH
1−2xO
1+x, have not been observed yet [
34].
Though not explicitly studied, several indications were reported for a significant phase width of the anion-disordered fluorite-type
REHO compounds: The samarium, dysprosium, and holmium representatives showed two distinct phases that differed in their lattice parameter
a in a synchrotron X-ray powder diffraction study (Sm: Δ
a/
a = 0.22%, Dy: Δ
a/
a = 0.09%, Ho: Δ
a/
a = 0.04%) [
12]. A greater discrepancy was also reported for two gadolinium compounds, although in different research groups (Δ
a/
a = 0.35%) [
12,
20]. Differences in similar magnitude were observed for the anion-ordered and disordered holmium compounds (Δ
a/
a = 0.65%) [
12,
42], whose anion order was verified by neutron diffraction measurements. The anion-disordered compound additionally showed a significant deviation of a 1:1:1 stoichiometry in the refinement of neutron diffraction data (
SOF(H) = 1 −
SOF(O) = 0.526(2)) [
12] (please note that lifting the restriction of full occupation of crystallographic sites (
SOF(H) +
SOF(O) = 1) enables further structural models in the refinement, e.g., an anion-deficient hydride oxide including vacancies). In a similar study of the anion-disordered erbium compound, such a deviation was not observed (
SOF(H) = 0.494(3),
SOF(O) = 0.505(1)) [
7]. Furthermore, several studies verify a broad compositional range of thin film rare earth hydride oxides [
26,
27].
The identification of such phase widths is an important issue, as it has a major impact on a compound’s properties, especially for ion conductivity. From a crystallographic point of view, the formation of hydrogen-rich phases
REH
1+2xO
1−x (
REX2+x;
X = H and O) demand the occupation of further crystallographic sites by anions as a full occupation of the tetrahedral interstices of the metal lattice by anions corresponds to a ratio
REX2. This is most probably the octahedral interstice, due to its size. In the oxygen-rich counterparts
REH
1−2xO
1+x (
REX2−x), voids must be formed. As the migration of anions in fluorite-related materials happens via octahedral interstices or voids [
7,
34,
43], their quantity directly affects the ionic transport.
The differentiation between anion-ordered and -disordered fluorite phases is of similar importance. A recent literature example observed a difference between conducting and isolating behavior, dependent on the anion ordering in Ba
2YHO
3 and Ba
2ScHO
3 [
9]. Unfortunately, a reliable differentiation between the anion-ordered and disordered cubic
REHO modifications (
Fm3m vs.
F43
m) is difficult by laboratory X-ray data and best performed with neutron diffraction analysis [
42]. In our previous study on HoHO [
42], the lattice parameter proved to be easily accessible and observable for differentiation here, which is why we wanted to expand our study to holmium’s nearest neighbors in the periodic table, dysprosium, and erbium in order to elaborate a simple tool for a general differentiation of ordered and disordered cubic
REHO compounds. Furthermore, the representatives of the smallest rare earth elements Sc, Tm, Yb, and Lu are still missing, though DFT calculations predicted their presence [
41,
42]. We therefore attempted the synthesis of LuHO by a different synthesis technique starting from LuH
3 and CaO, which was previously successful for YHO [
41].
Note on Nomenclature: Metal–hydrogen compounds are called metal hydrides. The natural isotopic mixture of hydrogen gas consists of 99.985% 1H (protium) + 0.015% 2H (deuterium, D). In this manuscript, the term metal hydride is used as a collective name including all isotopes, i.e., protides, deuterides, and tritides. They are only differentiated explicitly when isotope effects are important, for example in neutron diffraction experiments. This nomenclature is used accordingly for metal hydride oxides.
2. Materials and Methods
Synthesis. All manipulations were performed in an argon-filled glove box (Air Liquide, Düsseldorf, Germany; 99.999% purity), whose water and oxygen levels were kept below 1 ppm. DyHO, ErHO, and LuHO were obtained from the trihydrides and sesquioxides (
REH
3 +
RE2O
3 → 3
REHO) with an excess of hydride compensating its thermal decomposition, and LuHO and LuDO from the reaction of the trihydride (-deuteride) and calcium oxide (LuH
3 + CaO → LuHO + CaH
2) with a slight CaO excess. The sesquioxides (Kristallhandel Kelpin, Er
2O
3: 99.9%) were pretreated thermally prior to use at 1425 K for 6 h in a corundum crucible in air and transferred while hot into the glove box [
44]. The trihydrides were obtained from the hydrogenation of 2–5 g metal ingots (Dy, Er: Smart Elements, Vienna, Austria, 99.9%; Lu: Onyxmet, Olsztyn, Poland, 99.95%) with hydrogen (Air Liquide, Düsseldorf, Germany 99.9%) or deuterium gas (Air Liquide, Düsseldorf, Germany 99.8%) at 4–8 MPa gas pressure in sealed home-built autoclaves made from Inconel (Böhler L718V). Samples were initially heated to 725 K for 2 h and annealed subsequently for 48 h at 525 K. The thermal decomposition of lanthanide trihydrides under formation of H
2 gas requires the choice of a reaction vessel with sufficient material strength to endure the gas pressures and an aligned ratio of trihydride amount to container (gas) volume. For the synthesis of the hydride oxides, silica glass ampoules (10.5 mm inner diameter, 1.2 mm wall thickness) were cleaned with diluted nitric acid and acetone, stored at 450 K for several days, and transferred while hot into the glove box. The starting materials were mixed, ground, and filled into the silica glass ampoules, which were fused subsequently. For DyHO and ErHO, 1.8 g trihydride and 1.5 g oxide (molar ratio 2:1) were sealed in a tube of 20 cm length, heated with 50 K h
−1 to 1175 K, and annealed for 48 h before resuming to room temperature with 25 K h
−1. For the reaction LuH
3 + Lu
2O
3 → 3 LuHO, 200 mg trihydride and 220 mg oxide (molar ratio 2:1) were sealed in a tube of 7 cm length, heated with 100 K h
−1 to 1050 K, and resumed to 550 K with 10 K h
−1 before switching off the furnace. For the reaction LuH
3 + CaO → LuHO + CaH
2, 700 mg trihydride (2.0 g trideuteride) and 230 mg (700 mg) CaO (molar ratio 1:1.1) were sealed in silica glass tubes of 6 cm (20 cm) length, heated with 25 K h
−1 to 800 K, and annealed for 48 h (96 h) before switching off the furnace. For the hydride, this procedure was repeated after grinding, and the sample was washed by stirring for 20 min in 60 mL of a NH
4Cl solution in methanol in air, filtrated, rinsed twice with a total of 40 mL methanol, and vacuum dried before being transferring back into the glovebox.
Neutron powder diffraction (NPD) analysis. NPD data were collected on the HRPT high-resolution diffractometer at Paul-Scherrer Institut (PSI, Villigen, Switzerland) with a wavelength of 1.155 Å (DyHO, ErHO) and 1.494 Å (LuDO) [
45]. Specimen were filled in vanadium containers of 8 mm diameter in a helium glove box with controlled H
2O and O
2 impurities < 1 ppm, sealed with indium gaskets, and measured at ambient temperature. The high absorption of thermal neutrons of the dysprosium sample was addressed by longer counting times.
X-ray powder diffraction (XRPD) analysis. Specimen for XRPD experiments were mixed with powdered diamond to reduce X-ray absorption and filled into Lindemann glass capillaries of 0.2 mm diameter, which were subsequently sealed. They were analyzed by a Stoe STADI-P diffractometer (Stoe & Cie GmbH, Darmstadt, Germany) with Debye-Scherrer geometry and Ge(111) monochromatized Cu-Kα1 radiation. The data for cubic LuHO were collected in two experiments for small (20–92° 2θ) and large angles (89–128° 2θ).
Rietveld refinement. Powder diffraction data were refined with the Rietveld method [
46,
47] as implemented in the software GSAS-II version 4673 [
48] (NPD data) or Topas version 5 (Bruker AXS, Billerica, MA, USA, XRPD data) employing the fundamental parameter approach. Instrumental functions were provided by the PSI for the former and determined empirically on a Si NIST 640d reference material for the latter. The background was simulated with Chebychev type polynomials of second (LuDO), fourth (NPD of ErHO, LuDO; XRPD data), or fifth (NPD of DyHO) order and single line fits for the contribution of the X-ray capillary material. In each case, the zero-point error, scaling factors, lattice, profile, and positional parameters were refined in addition to the isotropic displacement factor
Biso, unless the phase ratio deceeded 10 wt%, in which case only the former four parameters were considered. For XRPD data, the
Biso values were corrected with an offset value
Boverall, which was refined from the diamond reflections using a literature value for diamond (
Biso(C) = 0.142 Ų [
49]). The neutron absorption of the dysprosium sample was considered by calculating the absorption parameter
µR with the FRM-II neutron calculator assuming an effective mass density of half the crystallographic mass density (
µR = 4.5, fixed during refinement) [
50]. It should be noted that GSAS-II regards the energy dependence of erbium’s coherent scattering length (
bc(Er) = 8.09 fm instead of 7.79 fm at 1.798 Å [
51,
52]).
Density functional theory (DFT) calculations. The density functional theory calculations were performed with the Vienna ab intio simulation package (VASP) version 5.4.4 [
53,
54] using projector augmented wave pseudopotentials (PAWs) [
55] from the VASP database. The
f electrons of lanthanides
Ln were described as part of the frozen core (
Ln_3). The
s and
p level electrons of the second highest principal quantum number were treated as semi-core states of Li, Ca, and Y (Li_sv, Ca_sv, Y_sv). For La, H, O, and F, the standard potentials were employed (La, H, O, F). The GGA-PBE method [
56] described the exchange correlation potential, and the Brillouin zone was integrated using the tetrahedron method with Blöchl corrections [
57]. The
k-grid was generated automatically and Γ-centered, ensuring a
k-point density of 0.03 Å
−1 in each direction (LiH, LiF, CaO 8 × 8 × 8; CaH
2 6 × 10 × 6;
REH
3 6 × 6 × 5;
RE2O
3 3 × 3 × 3; tetragonal
REHO 4 × 4 × 6; orthorhombic
REHO 4 × 9 × 6; cubic
REHO 6 × 6 × 6;
REOF 10 × 10 × 2). The cutoff energy was set to 800 eV. Structure optimizations were carried out in accurate precision mode and with full degrees of freedom regarding cell shape, cell volume, and atomic positions, using a conjugate gradient algorithm and converging forces to 10 µeV Å
−1 and electronic energies to 1 µeV. The initial crystal structures were obtained from the inorganic crystal structure database [
58] (
REH
3: YH
3 P3c1 deposition number 154809;
RE2O
3: Lu
2O
3 Ia3 194467;
REHO: LaHO
P4/
nmm 48122, YDO
Pnma 1903936, HoHO
F43
m 1993608, YOF
R3m 184004, LiH
Fm3m 61751, LiF
Fm3m 41409, CaH
2 Pnma 261185, CaO
Fm3m 163628). A- and B-type sesquioxides generally yielded higher absolute energies, which is why only the C-type structure was regarded. The total energies derived from the structure optimizations were used to calculate free reaction enthalpies Δ
rG and differences in free reaction enthalpies ΔΔ
rG.
4. Conclusions
We prepared five new rare earth hydride oxides, DyHO, ErHO, LuDO, as well as cubic and orthorhombic LuHO, and investigated their structure by neutron and X-ray powder diffraction. DyHO, ErHO, and LuHO crystallized in the anion-ordered modification with space group
F43
m, similar to the previously reported HoHO [
42]. LuHO and LuDO furthermore crystallized in the orthorhombic anti-LiMgN structure type. A comparison of the cubic rare earth hydride oxide’s lattice parameters
a showed its sensitivity to differentiate
REHO and
REH
1+2xO
1−x compounds, whereas the values of the latter were significantly larger than those extrapolated from the former. This differentiation is especially important in the research of their ionic conductivity. The lattice parameter can, however, not be used to distinguish between the cubic anion-ordered and disordered modifications of
REHO. Our study thus stresses the importance of a thorough understanding of the anionic substructure in the system
RE–H–O, which is more complex than previously assumed, though probably none of the reported substances is interesting for applications in ion conductors [
7] or catalysts [
12] due to the small cations with less efficient Coulomb shielding and thus small hydride ion diffusivity. Furthermore, DFT calculations supported our experimental findings: The compounds
REHO are more stable for the larger rare earth elements, whereas the sesquioxides become more stable than
REHO with decreasing cationic radius. For the smallest representatives including Lu, mild reaction conditions for the stabilization of kinetic products are thus necessary. Alternatively, a synthesis from the oxide fluorides
REOF might be successful for the thus far elusive Tm, Yb, and Sc compounds.