Synthesis and Crystal Structure Analysis of Some Aromatic Imines of Syringaldehyde
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental
2.2. Synthesis and Crystallization
2.3. Data Collection and Refinement
3. Results and Discussion
3.1. Synthesis and Spectroscopic Characterization
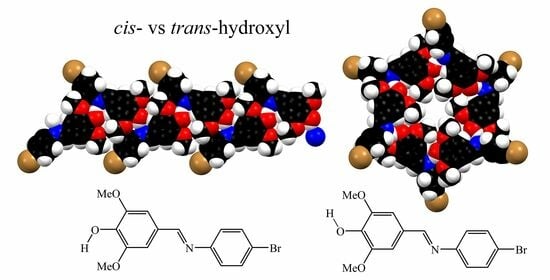
3.2. Crystal Structures
3.3. Hirshfeld Analysis
3.4. Hydrogen Bonding Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Leibigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Hamaker, C.G.; Halbach, D.P. Synthesis, structure, and characterization of some ruthenium arene complexes of N-(arylmethylene)-2-(methylthio)anilines and 2-(methylthio)aniline. Inorg. Chim. Acta 2006, 359, 846–853. [Google Scholar] [CrossRef]
- Pervaiz, M.; Sadiq, S.; Sadiq, A.; Younas, U.; Ashraf, A.; Saeed, Z.; Zuber, M.; Adnan, A. Azo-Schiff base derivatives of transition metal complexes as antimicrobial agents. Coord. Chem. Rev. 2021, 447, 214128. [Google Scholar] [CrossRef]
- Gonul, I.; Demirbag, B.; Ocakoglu, K.; Ayaz, F. Unique photodynamic antimicrobial Schiff bases and their copper complexes exert immunomodulatory activity on mammalian macrophages. J. Coord. Chem. 2020, 73, 2878–2888. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Bian, M.; Yang, Z.; Ma, X.; Liu, W. Pt(II) and Au(III) complexes containing Schiff-base ligands: A promising source for antitumor treatment. Eur. J. Med. Chem. 2021, 211, 113098. [Google Scholar] [CrossRef]
- Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Tereteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules 2021, 26, 2334. [Google Scholar] [CrossRef]
- Creighton, R.H.J.; McCarthy, J.L.; Hibbert, H. Aromatic Aldehydes from Spruce and Maple Woods. J. Am. Chem. Soc. 1941, 63, 312. [Google Scholar] [CrossRef]
- Guchu, E.; Diaz-Maroto, M.C.; Diaz-Maroto, I.J.; Vila-Lameiro, P.; Perez-Coello, M.S. Influence of the Species and Geographical Location on Volatile Composition of Spanish Oak Wood (Quercus petraea Liebl. and Quercus robur L.). J. Agric. Food Chem. 2006, 54, 3062–3066. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Hoffman, B.; Yang, J.; Soleas, G.J. Phenolic Constituents, Furans, and Total Antioxidant Status of Distilled Spirits. J. Agric. Food Chem. 1999, 47, 3978–3985. [Google Scholar] [CrossRef]
- Alcarde, A.R.; Souza, L.M.; Bortoletto, A.M. Formation of volatile and maturation-related congeners during the aging of sugarcane spirit in oak barrels. J. Inst. Brew. 2014, 120, 529–536. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Sriprasanthi, R.B.; Shamsudeen, S.; Adam, F.; Bhawani, S.A. A concise review of the natural existance, synthesis, properties, and applications of syringaldehyde. BioResources 2012, 7, 4377–4399. [Google Scholar] [CrossRef]
- Kuo, S.C.; Chung, H.H.; Huang, C.H.; Cheng, J.T. Decrease of Hyperglycemia by Syringaldehyde in Diabetic Rats. Horm. Metab. Res. 2014, 46, 8–13. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, M.F.; Chung, H.H.; Cheng, J.T. Antihyperglycemic Effect of Syringaldehyde in Streptozotocin-Induced Diabetic Rats. J. Nat. Prod. 2012, 75, 1465–1468. [Google Scholar] [CrossRef]
- Shahzad, S.; Mateen, S.; Mariyath, P.M.M.; Naeem, S.S.; Akhtar, K.; Rizvi, W.; Moin, S. Protective effect of syringaldehyde on biomolecular oxidation, inflammation and histopathological alterations in isoproterenol induced cardiotoxicity in rats. Biomed. Pharmacother. 2018, 108, 625–633. [Google Scholar] [CrossRef]
- Yancheva, D.; Velcheva, E.; Glavcheva, Z.; Stamboliyska, B.; Smelcerovic, A. Insights in the radical scavenging mechanism of syringaldehyde and generation of its anion. J. Molec. Struct. 2016, 1108, 552–559. [Google Scholar] [CrossRef]
- Stanikunaite, R.; Khan, S.I.; Trappe, J.M.; Ross, S.A. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle elaphomyces granulatus. Phytother. Res. 2009, 23, 575–578. [Google Scholar] [CrossRef]
- Aytac, S.; Gundogdu, O.; Bingol, Z.; Gulcin, İ. Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties. Pharmaceutics 2023, 15, 779. [Google Scholar] [CrossRef]
- McCrone, W.C. Polymorphism. In Physics and Chemistry of the Organic Solid State; Fox, D., Labes, M.M., Weissberger, A., Eds.; Interscience: New York, NY, USA, 1965; Volume 2, pp. 725–767. [Google Scholar]
- Bernstein, J.; Davey, R.J.; Henck, J.O. Concomitant polymorphs. Angew. Chem. Int. Ed. 1999, 38, 3440–3461. [Google Scholar] [CrossRef]
- Gong, N.; Hu, K.; Jin, G.; Du, G.; Lu, Y. Concomitant polymorphs of methoxyflavone (5-methyl-7-methoxyflavone). RSC Adv. 2016, 6, 38709–38715. [Google Scholar] [CrossRef]
- Kuś, P.; Borek, J.; Jones, P.G. Two concomitant polymorphs of N,N′-bis [4-(diethylamino)phenyl]terephthaldiamide. Acta Crystallogr. Sect. C Struct. Chem. 2010, C66, o93–o96. [Google Scholar] [CrossRef]
- Lee, E.H. A practical guide to pharmaceutical polymorph screening & selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar]
- Hamaker, C.G.; Maryashina, O.S.; Daley, D.K.; Wadler, A.L. Synthesis and Crystal Structures of Two Schiff Bases of 2-(Methylthio)aniline with Halogenated Salicylaldehydes. J. Chem. Crystallogr. 2010, 40, 34–39. [Google Scholar] [CrossRef]
- Goettler, P.E.; Hamaker, C.G. Crystal Structure Analysis of Two 4-Nitro-N-methylaniline Derivatives. J. Chem. Crystallogr. 2022, 52, 251–259. [Google Scholar] [CrossRef]
- Li, E.W.; Katinas, J.; Jones, M.A.; Hamaker, C.G. Structural characterization of naphthalene sulfonamides and a sulfonate ester and their in vitro efficacy against Leishmania tarentolae promastigotes. New J. Chem. 2021, 45, 4791–4801. [Google Scholar] [CrossRef]
- Bruker AXS Inc. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Hamaker, C.G.; Oberts, B.P. Synthesis and crystal structures of the bis-Schiff bases of 2-(methylthio)aniline with isophthaldehyde, terephthaldehyde, and para-diacetylbenzene. J. Chem. Crystallogr. 2006, 36, 735–742. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Asghari, J.; Fejfarová, K.; Dušek, M. 4-Chloro-N-(3,4,5-trimethoxybenzylidene)aniline Acta Crystallogr. Sect. E Crystallogr. Commun. 2009, E65, o253. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Weil, M.; Gotoh, K.; Ishida, H. 4-Bromo-N-(3,4,5-trimethoxybenzylidene)aniline. Acta Crystallogr. Sect. E Crystallogr. Commun. 2009, E65, o436. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Chernyshov, I.Y.; Ananyev, I.V.; Pidko, E.A. Revisiting van der Waals Radii: From Comprehensive Structural Analysis to Knowledge-Based Classification of Interatomic Contacts. ChemPhysChem 2020, 21, 370–376. [Google Scholar] [CrossRef]
























| Compound | Ia (Br-m) | Ib (Br-h) | II (OMe) | III (OH) |
|---|---|---|---|---|
| CCDC Deposit No. | 2277669 | 2320930 | 2277668 | 2277671 |
| Chemical formula | C15H14BrNO3 | C15H14BrNO3 | C16H17NO4 | C15H15NO4 |
| Mr | 336.18 | 336.18 | 287.30 | 273.28 |
| Crystal system, space group | Monoclinic, P21/c | Trigonal, | Monoclinic, P21/c | Monoclinic, P21 |
| Temperature (K) | 100 (2) | 100 (2) | 100 (2) | 100 (2) |
| a, b, c (Å) | 13.3573 (3) | 27.7665 (6) | 10.4238 (10) | 6.1284 (5) |
| 13.7075 (3) | 27.7665 (6) | 12.4291 (12) | 11.5549 (9) | |
| 15.6427 (4) | 9.6043 (3) | 13.1323 (13) | 9.6717 (7) | |
| a, β, γ (°) | 90 | 90 | 90 | 90 |
| 100.440 (1) | 90 | 122.2470 (10) | 98.233 (4) | |
| 90 | 120 | 90 | 90 | |
| V (Å3) | 2816.69 (11) | 6412.7 (3) | 1439.0 (2) | 677.82 (9) |
| Z | 8 | 18 | 4 | 2 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| µ (mm−1) | 2.925 | 2.891 | 0.096 | 0.098 |
| Crystal size (mm) | 0.22 × 0.16 × 0.12 | 0.50 × 0.04 × 0.04 | 0.33 × 0.26 × 0.18 | 0.14 × 0.10 × 0.08 |
| Diffractometer | Bruker APEX-II CCD | |||
| Absorption correction | Multi-scan SADABS | |||
| Tmin, Tmax | 0.59, 0.72 | 0.67, 0.89 | 0.95, 0.98 | 0.95, 0.99 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 71,268, 5987, 5627 | 40,757, 2832, 2633 | 21,013, 3053, 2826 | 19,802, 2938, 2855 |
| Rint | 0.0194 | 0.0251 | 0.0174 | 0.0244 |
| (sin θ/λ)max (Å−1) | 0.634 | 0.619 | 0.632 | 0.638 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0205, 0.0558, 1.081 | 0.0188, 0.0487, 1.047 | 0.0323, 0.0863, 1.034 | 0.0256, 0.0653, 1.047 |
| No. of reflections | 5987 | 2832 | 3053 | 2938 |
| No. of parameters | 369 | 185 | 194 | 189 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | |||
| Δρmax, Δρmin (e Å−3) | 0.882, −0.892 | 0.464, −0.179 | 0.273, −0.205 | 0.190, −0.180 |
| Bond | Ia | Ib | II | III |
|---|---|---|---|---|
| C6–N7 | 1.4262 (18), 1.4222 (18) | 1.4265 (19) | 1.4325 (13) | 1.4278 (19) |
| N7–C8 | 1.2852 (19), 1.2840 (19) | 1.281 (2) | 1.2828 (13) | 1.283 (2) |
| C8–C9 | 1.4633 (19), 1.4566 (19) | 1.464 (2) | 1.4678 (13) | 1.468 (2) |
| C12–O17 | 1.3611 (17), 1.3529 (17) | 1.3554 (18) | 1.3609 (12) | 1.363 (2) |
| Bond | Ia | Ib | II | III |
|---|---|---|---|---|
| C6–N7–C8 | 115.68 (12), 114.79 (12) | 116.88 (13) | 116.16 (9) | 117.50 (13) |
| N7–C8–C9 | 126.63 (13), 126.20 (13) | 124.55 (14) | 124.51 (9) | 123.26 (14) |
| C11–O15–C16 | 116.99 (12), 116.55 (11) | 116.80 (12) | 116.82 (8) | 116.73 (13) |
| C12–O17–H17 | 110.9 (15), 112.5 (17) | 110.0 (17) | 112.1 (12) | 108.5 (18) |
| C13–O18–C19 | 116.51 (11), 166.31 (11) | 116.76 (12) | 116.57 (8) | 117.04 (13) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| C14A–H14A…O17B i | 0.95 | 2.26 | 3.1771 (17) | 163 |
| C2A–H2A…O17A ii | 0.95 | 2.60 | 3.3113 (18) | 133 |
| O17A–H17A…N7B | 0.80 (2) | 2.22 (2) | 2.9442 (16) | 151.7 (19) |
| C14B–H14B…O17A | 0.95 | 2.36 | 3.2847 (17) | 166 |
| C1B–H1B…O18B iii | 0.95 | 2.55 | 3.4854 (18) | 169 |
| C2B–H2B…O17B iii | 0.95 | 2.53 | 3.2262 (19) | 130 |
| C4B–H4B…Br21 iv | 0.95 | 2.88 | 3.7816 (15) | 160 |
| O17B–H17B…N7A v | 0.76 (2) | 2.17 (2) | 2.8553 (17) | 152 (2) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| C14–H14…O17 i | 0.95 | 2.24 | 3.1680 (12) | 165 |
| C8–H8…O20 ii | 0.95 | 2.60 | 3.3906 (13) | 141 |
| C1–H1…O18 iii | 0.95 | 2.57 | 3.5027 (13) | 166 |
| C19–H19B…O15 i | 0.98 | 2.65 | 3.1313 (13) | 110 |
| O17–H17…N7 iv | 0.87 (2) | 2.10 (2) | 2.9010 (12) | 152.5 (17) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| C10–H10…O17 i | 0.95 | 2.36 | 3.271 (2) | 160 |
| O20–H20…N7 ii | 0.85 (3) | 1.92 (3) | 2.7693 (19) | 176 (3) |
| O17–H17…N7 iii | 0.81 (3) | 2.54 (3) | 3.0061 (18) | 117 (2) |
| O17–H17…O18 | 0.81 (3) | 2.21 (2) | 2.6432 (18) | 114 (2) |
| D–H…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| C14–H14…O17 i | 0.95 | 2.29 | 3.1785 (18) | 156 |
| C19–H19…O18 i | 0.98 | 2.52 | 3.1870 (19) | 125 |
| O17–H17…N7 ii | 0.75 (2) | 2.21 (2) | 2.8819 (17) | 150 (2) |
| O17–H17…O15 | 0.75 (2) | 2.28 (2) | 2.6659 (15) | 113 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamaker, C.G.; Germann, S.M. Synthesis and Crystal Structure Analysis of Some Aromatic Imines of Syringaldehyde. Crystals 2024, 14, 99. https://doi.org/10.3390/cryst14010099
Hamaker CG, Germann SM. Synthesis and Crystal Structure Analysis of Some Aromatic Imines of Syringaldehyde. Crystals. 2024; 14(1):99. https://doi.org/10.3390/cryst14010099
Chicago/Turabian StyleHamaker, Christopher G., and Stephan M. Germann. 2024. "Synthesis and Crystal Structure Analysis of Some Aromatic Imines of Syringaldehyde" Crystals 14, no. 1: 99. https://doi.org/10.3390/cryst14010099
APA StyleHamaker, C. G., & Germann, S. M. (2024). Synthesis and Crystal Structure Analysis of Some Aromatic Imines of Syringaldehyde. Crystals, 14(1), 99. https://doi.org/10.3390/cryst14010099