Synthesis, Crystal Structure and Luminescent Properties of 2D Zinc Coordination Polymers Based on Bis(1,2,4-triazol-1-yl)methane and 1,3-Bis(1,2,4-triazol-1-yl)propane
Abstract
:1. Introduction
2. Results and Discussion
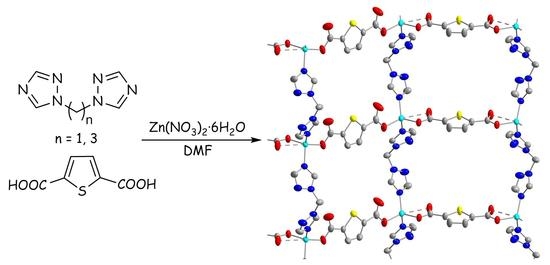
2.1. Synthesis of Coordination Polymers
2.2. Crystal Structures
2.2.1. Crystal Structure of Polymer [Zn(tdc)(btrm)]∙nDMF (1)
2.2.2. Crystal Structure of Polymer [Zn(tdc)(btrp)]∙nDMF (2)
2.3. Thermal Analysis
2.4. Luminescent Properties
3. Experimental Section
3.1. Materials and Methods
3.2. X-ray Structure Determination
3.3. Synthesis of Compounds
3.3.1. Synthesis of [Zn(btrm)(tdc)]·nDMF (1)
3.2.2. Synthesis of [Zn(btrp)(tdc)]·nDMF (2)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Application of metal-organic frameworks. Polym. Int. 2017, 66, 731–744. [Google Scholar] [CrossRef]
- You, A.; Li, Y.; Zhang, Z.M.; Zou, X.Z.; Gu, J.Z.; Kirillov, A.M.; Chen, J.W.; Chen, Y.B. Novel metal-organic and supramolecular 3D frameworks constructed from flexible biphenyl-2,5,3′-tricarboxylate blocks: Synthesis, structural features and properties. J. Mol. Struct. 2017, 1145, 339–346. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Cai, Y.; Liu, Y.; Liang, X.-X.; Kirillov, A.M. New lanthanide 2D coordination polymers constructed from a flexible ether-bridged tricarboxylate block: Synthesis, structures and luminescence sensing. Inorg. Chim. Acta 2018, 469, 98–104. [Google Scholar] [CrossRef]
- Huang, W.; Pan, F.; Liu, Y.; Huang, S.; Li, Y.; Yong, J.; Li, Y.; Kirillov, A.M.; Wu, D. An efficient blue-emissive metal-organic framework (MOF) for lanthanide-encapsulated multicolor and stimuli-responsive luminescence. Inorg. Chem. 2017, 56, 6362–6370. [Google Scholar] [CrossRef] [PubMed]
- Semitut, E.; Komarov, V.; Sukhikh, T.; Filatov, E.; Potapov, A. Synthesis, crystal structure and thermal stability of 1D linear silver(I) coordination polymers with 1,1,2,2-Tetra(pyrazol-1-yl)ethane. Crystals 2016, 6, 138. [Google Scholar] [CrossRef]
- Barsukova, M.; Goncharova, T.; Samsonenko, D.; Dybtsev, D.; Potapov, A. Synthesis, crystal structure, and luminescent properties of new zinc(II) and cadmium(II) metal-organic frameworks based on flexible bis(imidazol-1-yl)alkane ligands. Crystals 2016, 6, 132. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R. Coordination polymers and metal-organic frameworks: Materials by design. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 375. [Google Scholar] [CrossRef] [PubMed]
- Dybtsev, D.N.; Sapianik, A.A.; Fedin, V.P. Pre-synthesized secondary building units in the rational synthesis of porous coordination polymers. Mendeleev Commun. 2017, 27, 321–331. [Google Scholar] [CrossRef]
- An, Z.; Wang, J. Gas adsorption and luminescent properties of a porous pcu-type Zn(II) coordination polymer. Synth. React. Inorg. Met. Nano-Met. Chem. 2016, 46, 1810–1814. [Google Scholar] [CrossRef]
- An, Z.; Zhu, L. A new luminescent Zn(II) coordination polymer with eightfold interpenetrated ths topology. Synth. React. Inorg. Met. Nano-Metal Chem. 2016, 46, 1367–1370. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Qin, J. Porous pcu-type Zn(II) framework material with high adsorption selectivity for CO2 over N2. J. Mol. Struct. 2016, 1107, 66–69. [Google Scholar] [CrossRef]
- Erer, H. Effect of O and S heteroatom containing heterocyclic dicarboxylates in the structural diversity of cadmium(II) coordination polymers with flexible 1-substituted (1,2,4-triazole) ligand. Polyhedron 2015, 102, 201–206. [Google Scholar] [CrossRef]
- Li, X.; Zhou, P.; Dong, Y.; Liu, H. Structural diversity of a series of 2D Zn(II) coordination polymers tuned by different dicarboxylic acids ligands. J. Inorg. Organomet. Polym. Mater. 2015, 25, 650–656. [Google Scholar] [CrossRef]
- Sapchenko, S.A.; Samsonenko, D.G.; Fedin, V.P. Synthesis, structure and luminescent properties of metal-organic frameworks constructed from unique Zn- and Cd-containing secondary building blocks. Polyhedron 2013, 55, 179–183. [Google Scholar] [CrossRef]
- Sapchenko, S.A.; Saparbaev, E.S.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Synthesis, structure, and properties of a new layered coordination polymer based on Zinc(II) carboxylate. Russ. J. Coord. Chem. 2013, 39, 549–552. [Google Scholar] [CrossRef]
- Xie, W.; He, W.-W.; Du, D.-Y.; Li, S.-L.; Qin, J.-S.; Su, Z.-M.; Sun, C.-Y.; Lan, Y.-Q. A stable Alq3@MOF composite for white-light emission. Chem. Commun. 2016, 52, 3288–3291. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.-Y.; Dai, M.; Young, D.J.; Ren, Z.-G.; Lang, J.-P. Luminescent Zn(II) coordination polymers for highly selective sensing of Cr(III) and Cr(VI) in water. Inorg. Chem. 2017, 56, 4668–4678. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-H.; Qin, C.; Ding, Y.; Wu, H.; Shao, K.-Z.; Su, Z.-M. Pillared metal organic frameworks for the luminescence sensing of small molecules and metal ions in aqueous solutions. Dalton Trans. 2015, 44, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, D.; Zhang, X.; Ma, J.; Liu, L.; Xu, X. Preparation, structure and photocatalysis of metal-organic frameworks derived from aromatic carboxylate and imidazole-based ligands. J. Coord. Chem. 2016, 69, 985–995. [Google Scholar] [CrossRef]
- Zhao, S.; Li, M.; Shi, L.-L.; Li, K.; Li, B.-L.; Wu, B. Syntheses, structures and photocatalytic properties of three copper(II) coordination polymers. Inorg. Chem. Commun. 2016, 70, 185–188. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, C.; Zheng, X.; Tian, Z.; Wen, L.; Qu, H.; Li, D. New metal-organic frameworks based on 2,5-thiophenedicarboxylate and pyridine- or imidazole-based spacers: Syntheses, topological structures, and properties. Dalton Trans. 2013, 42, 16375–16386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zheng, T.-R.; Zhang, Y.-Q.; Lv, X.-X.; Li, B.-L.; Zhang, Y. Syntheses, structures and photocatalytic properties of a series of cobalt coordination polymers based on flexible bis(triazole) and dicarboxylate ligands. Polyhedron 2017, 121, 61–69. [Google Scholar] [CrossRef]
- Erer, H.; Yeşilel, O.Z.; Arıcı, M. A Series of zinc(II) 3D→3D interpenetrated coordination polymers based on thiophene-2,5-dicarboxylate and bis(Imidazole) derivative linkers. Cryst. Growth Des. 2015, 15, 3201–3211. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Liu, W.; Cao, Z.; Ren, Y.; Zhou, Q.; Zhang, L. Two new luminescent Zn(II) coordination polymers with different interpenetrated motifs. J. Mol. Struct. 2015, 1099, 49–53. [Google Scholar] [CrossRef]
- Sun, D.; Xu, M.-Z.; Liu, S.-S.; Yuan, S.; Lu, H.-F.; Feng, S.-Y.; Sun, D.-F. Eight Zn(II) coordination networks based on flexible 1,4-di(1H-imidazol-1-yl)butane and different dicarboxylates: Crystal structures, water clusters, and topologies. Dalton Trans. 2013, 42, 12324–12333. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.-P.; Chang, X.-H.; Ma, L.-F.; Wang, L.-Y. Four d10 metal coordination polymers based on bis(2-methyl imidazole) spacers: Syntheses, interpenetrating structures and photoluminescence properties. RSC Adv. 2014, 4, 60883–60890. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Wang, M.-Y.; Li, Q.-T.; Qian, B.-H.; Yang, X.-J.; Xu, X.-Y. Hydrothermal synthesis, crystal structure, and luminescent properties of two Zinc(II) and Cadmium(II) 3D metal-organic frameworks. Zeitschrift für Anorganische und Allgemeine Chemie 2013, 639, 826–831. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, Y. Two double and triple interpenetrated Cd(II) and Zn(II) coordination polymers based on mixed O- and N-donor ligands: Syntheses, crystal structures and luminescent properties. J. Mol. Struct. 2016, 1103, 56–60. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Cui, Y.-F.; Li, B.-L.; Li, H.-Y. A polythreading coordination array formed from 2D grid networks and 1D chains. CrystEngComm 2011, 13, 3342–3344. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Li, J.; Ma, J.-F.; Ma, J.-C.; Yang, J. A series of 1D, 2D and 3D coordination polymers based on a 5-(benzonic-4-ylmethoxy)isophthalic acid: Syntheses, structures and photoluminescence. CrystEngComm 2012, 14, 169–177. [Google Scholar] [CrossRef]
- Kan, W.-Q.; Ma, J.-F.; Liu, B.; Yang, J. A series of coordination polymers based on 5,5’-(ethane-1,2-diyl)-bis(oxy)diisophthalic acid and structurally related N-donor ligands: Syntheses, structures and properties. CrystEngComm 2012, 14, 286–299. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Hou, C.-T.; Song, J.-J.; Deng, Y.; Li, L.; Ng, S.W.; Diao, G.-W. Temperature and auxiliary ligand-controlled supramolecular assembly in a series of Zn(II)-organic frameworks: Syntheses, structures and properties. CrystEngComm 2012, 14, 590–600. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, X.; Wang, J.; Yang, Z.; Li, B.L.; Wu, B. Two unusual 3D and 2D zinc coordination polymers containing 2D or 1D [Zn2(btec)]n based on flexible bis(triazole) and rigid benzenetetracarboxylate co-ligands. Inorg. Chem. Commun. 2012, 26, 37–41. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, Y.; Jiang, N.; Li, B.; Zhou, D.; Fu, H.; Wang, N. Hydrothermal assembly of two new 3D Zinc(II) pcu nets: Coordination chemistry, crystal structures, and fluorescence properties. Zeitschrift für Anorganische und Allgemeine Chemie 2015, 641, 699–703. [Google Scholar] [CrossRef]
- Wang, J.; Qian, X.; Cui, Y.-F.; Li, B.-L.; Li, H.-Y. Syntheses, structures, and luminescence of three 4-connected zinc coordination polymers with bis(1,2,4-triazol-1-yl)propane and benzenebiscarboxylate. J. Coord. Chem. 2011, 64, 2878–2889. [Google Scholar] [CrossRef]
- Yan, Z.-H.; Han, L.-L.; Zhao, Y.-Q.; Li, X.-Y.; Wang, X.-P.; Wang, L.; Sun, D. Three mixed-ligand coordination networks modulated by flexible N-donor ligands: Syntheses, topological structures, and temperature-sensitive luminescence properties. CrystEngComm 2014, 16, 8747–8755. [Google Scholar] [CrossRef]
- Tian, L.; Niu, Z.; Yang, N.; Zou, J.-Y. Crystal structures and luminescent properties of zinc(II) and cadmium(II) compounds constructed from 5-sulfoisophthalic acid and flexible bis-triazole ligands. Inorg. Chim. Acta 2011, 370, 230–235. [Google Scholar] [CrossRef]
- Hong-Bing, Y.; Jian-Ge, W. Crystal structure of catena-[(μ2-5-methylisophthalato)(μ2-1,3-bis-(1,2,4- triazol-1-yl)propane)]Zinc(II)dihydrate, [Zn(C9H6O4)(C7H10N6)]·2H2O, C16H20N6O6Zn. Z. Krist.-New Cryst. Struct. 2012, 227, 457. [Google Scholar]
- Han, M.-L.; Chang, X.-H.; Feng, X.; Ma, L.-F.; Wang, L.-Y. Temperature and pH driven self-assembly of Zn(II) coordination polymers: Crystal structures, supramolecular isomerism, and photoluminescence. CrystEngComm 2014, 16, 1687–1695. [Google Scholar] [CrossRef]
- Tian, L.; Yang, N.; Zhao, G. Syntheses, structures, and luminescent properties of zinc(II) complexes assembled with aromatic polycarboxylate and 1,3-bis(1,2,4-triazol-1-yl)propane. Inorg. Chem. Commun. 2010, 13, 1497–1500. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Yue, F.-X.; Yu, X.-Y.; Gu, L.-L.; Zhang, H.; Chen, X. A series of entangled ZnII/CdII coordination polymers constructed from 1,3,5-benzenetricarboxylate acid and flexible triazole ligands. CrystEngComm 2013, 15, 8116. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Tao, C.-Z.; Zhang, D.-E.; Ma, J.-J.; Liu, L.; Tong, Z.-W.; Yu, X.-Y. Three new three dimensional Zn(II)-benzenetetracarboxylate coordination polymers: Syntheses, crystal structures and luminescent properties. Polyhedron 2017, 123, 69–74. [Google Scholar] [CrossRef]
- Semitut, E.Y.; Sukhikh, T.S.; Filatov, E.Y.; Anosova, G.A.; Ryadun, A.A.; Kovalenko, K.A.; Potapov, A.S. Synthesis, crystal structure, and luminescent properties of novel zinc metal-organic frameworks based on 1,3-Bis(1,2,4-triazol-1-yl)propane. Cryst. Growth Des. 2017, 17, 5559–5567. [Google Scholar] [CrossRef]
- Feng, W.; Chang, R.N.; Wang, J.Y.; Yang, E.C.; Zhao, X.J. Four 1,3-bis(1,2,4-triazol-1-yl)propane-based metal complexes tuned by competitive coordination of mixed ligands: Synthesis, solid structure, and fluorescence. J. Coord. Chem. 2010, 63, 250–262. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, K.; Yang, Y.; Li, B.-L.; Zhang, Y. Syntheses and structures of three zinc coordination polymers with 1-D zigzag chain, double chain, and triple chain. J. Coord. Chem. 2009, 62, 2358–2366. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, L.L.; Shi, Z.-Q.; Shang, J.-J.; Wu, X.H.; Wang, L.-Y.; Zhou, J.G. Synthesis, crystal structure, and luminescence property of a new Zinc(II) complex with schiff-base containing triazole propane ancillary ligand. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 1093–1098. [Google Scholar] [CrossRef]
- Yin, G.; Zhang, Y.; Li, B.; Zhang, Y. Syntheses, structures and luminescent properties of a dimer and an one-dimensional chain coordination polymer with the flexible bis(triazole) and hydroxybenzoate ligands. J. Mol. Struct. 2007, 837, 263–268. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Tian, L. Poly[hemi(hexaaquazinc) [[μ2-1,3-bis(1,2,4-triazol-1-yl)methane](μ2-5-sulfonatobenzene-1,3-dicarboxylato)zinc] sesquihydrate]. Acta Crystallogr. E 2011, 67, m950–m951. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y.; Zhu, M.-S.; Yin, T.-T.; Meng, Y.-S.; Wu, Z.-Q.; Zhang, Y.-Q.; Gao, S. Cobalt(II) coordination polymer exhibiting single-ion-magnet-type field-induced slow relaxation behavior. Inorg. Chem. 2015, 54, 3716–3718. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A. Synthesis, crystal structure and hirshfeld topology analysis of polymeric Silver(I) complex with s-triazine-type ligand. Crystals 2017, 7, 160. [Google Scholar] [CrossRef]
- PCPDFWin, Version 1.30, Swarthmore: Swarthmore, PA, USA, 1997.
- APEX2, Version 2.0, SAINT, Version 8.18c, and SADABS, Version 2.11, Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2000–2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]







| 1 | 2 | Btrm | Btrp | |
|---|---|---|---|---|
| Ex, nm | 330 | 375 | 330 | 260 br |
| Em, nm | 410 (sh), 430, 460 (sh) | 440 | 440 | 410 |
| QY | 0.1 | <0.005 1 | 0.03 | 0.04 |
| Parameter | Compound 1 | Compound 2 |
|---|---|---|
| Empirical formula | C11H8N6O4SZn | (C13H12N6O4SZn)·0.5(C3H7NO) |
| Formula weight | 385.66 | 450.26 |
| Crystal system | orthorhombic | monoclinic |
| Space group | Pbcm | P21/c |
| Unit cell dimensions a, Å | 9.3189(3) | 6.4235(2) |
| b, Å | 11.5387(3) | 15.4396(5) |
| c, Å | 37.2569(11) | 19.2190(7) |
| β, o | 90.7550(10) | |
| Volume, Å3 | 4006.2(2) | 1905.90(11) |
| Z | 8 | 4 |
| Density (calcd.), g·cm−3 | 1.279 | 1.569 |
| F(000) | 1552 | 920 |
| Abs. coefficient, mm−1 | 1.352 | 1.436 |
| Crystal size, mm3 | 0.18 × 0.17 × 0.08 | 0.40 × 0.30 × 0.10 |
| 2θmax, o | 51.60 | 51.60 |
| Index range | −11 ≤ h ≤ 7 −14 ≤ k ≤ 14 −45 ≤ l ≤ 45 | −7 ≤ h ≤ 7 −18 ≤ k ≤ 18 −23 ≤ l ≤ 23 |
| Reflections collected | 42,882 | 29,130 |
| Independent reflections | 3900 [R(int) = 0.0416] | 3405 [R(int) = 0.0381] |
| Completness to 2θ = 50.5, % | 99.8 | 99.4 |
| Reflections, I ≥ 2σ(I) | 3201 | 3405 |
| Parameters | 210 | 244 |
| Final R indices [I > 2σ(I)] | R1 = 0.0356 wR2 = 0.0836 | R1 = 0.0356 wR2 = 0.1064 |
| R indices (all data) | R1 = 0.0470 wR2 = 0.0871 | R1 = 0.0378 wR2 = 0.1084 |
| GoF | 1.048 | 1.060 |
| Residual electron density (min/max, e/Å3) | −0.282/0.310 | −0.613/0.952 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semitut, E.; Sukhikh, T.; Filatov, E.; Ryadun, A.; Potapov, A. Synthesis, Crystal Structure and Luminescent Properties of 2D Zinc Coordination Polymers Based on Bis(1,2,4-triazol-1-yl)methane and 1,3-Bis(1,2,4-triazol-1-yl)propane. Crystals 2017, 7, 354. https://doi.org/10.3390/cryst7120354
Semitut E, Sukhikh T, Filatov E, Ryadun A, Potapov A. Synthesis, Crystal Structure and Luminescent Properties of 2D Zinc Coordination Polymers Based on Bis(1,2,4-triazol-1-yl)methane and 1,3-Bis(1,2,4-triazol-1-yl)propane. Crystals. 2017; 7(12):354. https://doi.org/10.3390/cryst7120354
Chicago/Turabian StyleSemitut, Evgeny, Taisiya Sukhikh, Evgeny Filatov, Alexey Ryadun, and Andrei Potapov. 2017. "Synthesis, Crystal Structure and Luminescent Properties of 2D Zinc Coordination Polymers Based on Bis(1,2,4-triazol-1-yl)methane and 1,3-Bis(1,2,4-triazol-1-yl)propane" Crystals 7, no. 12: 354. https://doi.org/10.3390/cryst7120354
APA StyleSemitut, E., Sukhikh, T., Filatov, E., Ryadun, A., & Potapov, A. (2017). Synthesis, Crystal Structure and Luminescent Properties of 2D Zinc Coordination Polymers Based on Bis(1,2,4-triazol-1-yl)methane and 1,3-Bis(1,2,4-triazol-1-yl)propane. Crystals, 7(12), 354. https://doi.org/10.3390/cryst7120354