1. Introduction
Research on poly(trimethylene terephthalate) has been rapidly growing since the recent technology development advancing the production of bio-based 1,3-propanediol (PDO). The low-cost production of bio-based PTT with excellent physical properties like poly(ethylene terephthalate) (PET) as well as favorable processing-related properties comparable to polybutylene terephthalate (PBT), may result in it becoming the most promising bio-based polyester [
1]. Poly(trimethylene terephthalate) (PTT) is an achiral aromatic polyester that has three methylene groups arranged in gauche-gauche conformation in its alkylene moiety. Unlike PET and PBT, PTT exhibits large and well-defined banded spherulites at a relatively fast crystallization time when melt-crystallized in a film [
2,
3,
4,
5,
6,
7,
8,
9]. Furthermore, the terephthloyl moiety makes PTT a highly birefringent material, allowing its spherulites to appear colorful under a polarized optical microscope. With all these characteristic features, PTT is a good candidate for the study of polymer crystallization and self-assembly. Thus, in this paper, the works on PTT crystallization, including its kinetics [
10,
11], intrinsic birefringence [
12,
13,
14], as well as its spherulitic morphologies, particularly its banded spherulite structures and formations [
2,
3,
4,
5,
6,
7,
8,
9] are reviewed in order to provide insight into the real lamellar structures in banded spherulites crystallized from melting.
Banded spherulites are unique phenomena that can be found in the crystallization of substances, such as polymers, small compounds, or the combination of the two. Periodic changes of height profile and birefringence along the radial directions make banded spherulites more attractive than other spherulitic morphologies. Due to their complex yet systematic arrangements, banded spherulites have received considerable interests from both scientists and engineers. For almost a century, great efforts have been taken to investigate the origins and formation mechanisms of banded spherulites. At the early phase of the investigation, using polyethylene spherulites as a model, Keller explained that the phenomenon of banded spherulites originated from continuous twisting of lamellae along the radial direction [
15,
16,
17]. Later on, through detailed microscopy studies, Bassett, et al. showed that the banded spherulites of polyethylene consisted of untwisted S- or C-shaped lamellae [
18,
19].
For models of continuous lamellar twisting, there are four main driving forces that have been considered: anisotropic surface stresses [
20,
21,
22,
23,
24,
25,
26,
27]; screw dislocations [
28,
29]; thermal, concentration, and/or mechanical fields formed around the growing crystals [
30,
31,
32]; or autodeformation, the compositional heterogeneities within sub-individuals that induce mismatch or heterogeneous stresses of twist moment [
33]. Classical views on twisting were mainly based on the observation of thin films, except for Lustiger et al. [
25] who carried out preliminary work of dissecting the bulk specimen and exposed the three-dimensional (3-D) interior of banded spherulite of polyethylene. They suggested that the special lamellar shapes are not necessarily intrinsic to the lamellar profile, but arise due to geometrical effects as the lamellae project onto the surface at various angles.
Regarding the lamellar twisting model, however, there are several issues that could not be settled. The lamellae may twist during their growth and twisted lamellae can be found in any type of spherulite, not only in banded ones. Furthermore, with the advancement in characterizing technology, discrepancies between the pitches of twisted lamella and the optical band spacing are being considered against the models of continuous twisting [
2,
3,
34,
35]. In the case of classical polyethylene (PE), the lamellae twist/spiral is at 100 nm pitch [
34], while the optical banding pitch for PE is about 4 μm; thus, the discrepancy between transmission electron microscopy (TEM) and polarizing optical microscopy (POM) evidence for PE is 40 times. In the case of PTT, the discrepancy between TEM and POM evidence for lamellae spiral and optical banding pitch for their banded spherulite is up to 50 times [
2,
3].
In more recent years, novel and revolutionary models on the formation mechanisms and lamellar assembly of polymer spherulites have been proposed by Woo, et al. [
36,
37,
38,
39,
40,
41,
42]. Solid evidence of interior lamellar assembly in correspondence to optical band spacing, should be taken into account when discussing banded spherulites. Various approaches such as the use of additional diluents (either amorphous or crystalline polymer) and/or etching agents have been conducted to enhance the contrast of the interior crystal arrangement. It cannot be denied that the information about the true inner anatomy hidden under the top surface of the spherulite obtained by the interior dissection approach is necessary to provide a complete picture for the arguments.
PTT, either neat or in copolymerization with other polymers, is capable of forming multiple types of banded spherulites, not only concentric-ring but also spiral banded spherulites [
3,
41,
42]. The spiral banded spherulite may have one, two, or even more growing arms, separating one band from the others. Using the existing mechanisms to explain the growth of these banded spherulites, however, is still a considerable oversimplification of the real issues. Thus, this mini review aims to compile the arguments on the origin and formation mechanism of PTT banded spherulites and to give insight on the issues in further depth.
3. Crystal and Thermal Analyses
Although the crystal thickness resulting from isothermal crystallization, obtained from small-angle X-ray scattering (SAXS) data analyses, remains almost constant [
44], the melting behavior of the semicrystalline PTT is however quite complex [
4,
44,
45]. PTT exhibits multiple endothermic melting peaks when isothermally crystallized into banded spherulites [
4,
45]. To rationalize the appearance of multiple endothermic peaks, some investigators proposed the so-called melting-recrystallization model, assuming that the original imperfect/thin crystals could be melted and recrystallized into better perfection crystallites during heating. More recently, Ivanov, et al. [
44], through the examination of the differential size distribution function normalized by the wide-angle X-ray scattering (WAXS) crystallinity index, revealed that the crystals squeezed between the thinnest amorphous layers melt first. Thus, the melting point of the crystals does not solely depend on their thickness, but is also affected by the adjacent amorphous regions. During melting, the constraints on the crystals gradually relax, which increases the melting point of the remaining crystals.
3.1. During Annealing
Wu, et al. [
4] investigated the correlation between melting behavior and banded spherulites in PTT. Differential scanning calorimetry (DSC) characterization was performed on PTT samples crystallized at 190 °C for various annealing times (
tc) by scanning at various heating rates.
Figure 3 shows the representative DSC traces revealing three endothermic melting peaks (labeled as P1, P2, and P3) exhibited by PTT crystallized at 190 °C. The upper peaks P2 and P3 merge gradually with the increasing crystallization time. Interestingly, the increasing of annealing time and heating rate influence P1 and P2 but not P3. Thus, they suggest that P1 and P2 crystal entities distinctly and discreetly pre-exist upon annealing at 190 °C, while P3 crystal entities are generated by re-crystallization of molten P1 crystal entities upon DSC scanning.
The author also investigated the spherulitic morphology of the melt-crystallized PTT under POM. In order to understand the melting behavior of the crystallized PTT, they also examined the effect of post-crystallization annealing at 225 °C (above P1 and P2) on the 190 °C-crystallized banded spherulites.
Figure 4 shows the POM micrographs of 190 °C-crystallized PTT spherulites annealed at 225 °C for various holding times from 1 to 120 min. With the increasing holding times, the orange ring pattern was significantly enhanced in intensity. The post-crystallization annealing at a higher temperature induced more lamellar reorganization and reorientation. From this observation, the authors suggested that P3 belongs to the thickened lamellae that are produced from reorganization and reorientation during annealing/relaxing.
3.2. Banded Spherulites upon Melting
Melting of the crystallized PTT spherulites upon slow heating may generate useful information about the lamellae types corresponding to various colored bands. Chen, et al. [
5] observed the gradual fading interference colors during heating of the crystallized PTT samples to their melting point.
Figure 5 shows an in-situ POM observation on the 150 °C-crystallized PTT spherulites during the heating process from 195 °C to 225 °C. When heated to 205 °C, spherulites did not show an obvious color change, however, with further increasing temperature, the interference colors changed gradually from cyan–purple (the highest retardation value) to magenta–orange, to orange–yellow, to yellow–white, then to white–black, before all spherulites disappeared in a black color when all the crystals became fully molten and amorphous (melt-state) at high enough temperatures. The lamellae gradually swell and become less ordered during heating, thus the thickness of ordered crystals is indeed sensitively related to the interference colors. Prior to complete melting of PTT crystals at
T = 222 °C, the extinction bands in spherulites became almost invisible, although the spherulite frames were still intact. Spherulites completely disappeared at
T = 225 °C. When the temperature was increased to a certain melting point, the less perfect crystal lamellae melted first, then the crystal thickness decreased gradually, hence the retardation value was reduced gradually and the interference colors also changed gradually.
4. Top-Surface Banded Structures vs. Birefringence Patterns
In this section, the discussion will be focused on the observations of multiple banded structures (concentric-ring, single-spiral, and double-spiral) on the top surface of PTT spherulites as have been reported by Wang, et al. [
3] and Woo, et al. [
42]. Woo, et al. further conducted POM and AFM observations on PTT banded spherulites to examine the differences and similarities of these structures; results are shown in
Figure 6. Analyses on their interference colors, phase and height profiles as well as in-situ melting and crystallization processes verified several highlighted results. The interesting interior morphology features in PTT spherulites are: (1) A concentric-ring banded spherulite (
Figure 6a) has initial sheaf-like crystals equally grown in all radial directions without obstacle, with a core diameter equaling its band spacing. Secondly; (2) A spiral banded spherulite has its initial grown sheaf-like crystals curved around its long axis, making an S-shape. When both arms of the S-shaped crystal are equally grown, a double spiral banded spherulite (
Figure 6c) appears where the core diameter is roughly double the band spacing. When one of the arms becomes retarded or grows much slower than the other, a single spiral banded spherulite (
Figure 6b) appears, where the core diameter is exactly equal to the band spacing. In summary, the size of the core diameter of the central nuclei region tends to determine or correlate with the patterns of spiral patterns (single spiral vs. double spirals) later-on. Thirdly; (3) the growing arms consist of thicker crystal lamellae that are initially aligned with their long axes parallel to the spiral direction and gradually become oriented in the perpendicular direction as the spiral wave (i.e., in the radial direction) propagates toward the outer edge. Similar spiral crystal growth was previously reported by Okabe, et al. in the crystallization of poly(vinylidene fluoride) blend with poly(vinyl acetate) [
46].
Additionally, there are three subsidiary key points to be summarized. First, the following order is observed of the surface topographical slope, of concentric-ring < double-spiral < single-spiral banded spherulite. Secondly, the zoomed-in atomic force microscopy (AFM) images of the spiral banded spherulites enclosed in the discussion show that the crystal plates align themselves correspondingly to the growing arms which apparently differ from the monotonous twist in the radial direction from a common center as proposed previously by Ho et al. [
2]. The area between the growing arms is filled with fibril lamellae that align parallel to the spiral direction. Finally, these alternating alignments of the crystal bands in a circular/spiral segment supposedly make the banded spherulite appear in alternating colors under POM. Woo, et al. [
41] also verified that the area of fibril lamellae corresponds to the lower-interference-colored [weaker birefringence] band while the area of the growing arm(s) corresponds to higher-interference-colored band(s) with stronger birefringence, which is in contrast to that reported previously by Chuang, et al. [
13].
5. Banded Structures and Origins
Ho, et al. [
2] attempted to discuss the origins of PTT banded spherulite by following the lamellar twisting model originally suggested by Keith and Padden [
22,
23,
24]. They claimed that the periodic thickness variation (wavelike morphology) observed in PTT corresponded well to the periodic extinction of banded spherulites which is attributed to the lamellar twisting along the spherulitic radials. The shallow C-shaped and S-shaped textures in the crest regions of the TEM images are attributed to the shadowed effect of the incomplete lamellar twisting due to the thickness limitation.
Figure 7 show the schematic representation of (a) the lamellar geometry and (b) the twining mechanism of the intra-lamellar model in PTT proposed by Ho, et al. They illustrated their model on the basis of the geometry of single-crystal lamellae which have their a axes close to the radial directions of the spherulite (
Figure 7a). The non-orthogonal relationship between chain stems to the fold surfaces develop the unbalanced surface stresses which induce lamellar twisting shown in
Figure 7b. This interpretation was then adopted by many other investigators to explain their observation results on PTT banded spherulites [
5,
6,
7,
8,
9].
Recently, Rosenthal, et al. [
8] revisited the model of Keith and Padden [
22,
23,
24]. They attempted to find the origin of crystal lamellae twisting by exploring the microstructure of PTT banded spherulites crystallized at 170 °C from approximately 30 µm-thickness sample (can be considered as a bulk sample) using microbeam X-ray diffraction.
Figure 8 shows the schematics illustrating (a) the opposite sense of lamellar twisting along the negative and positive growth directions as well as (b) the orientation of the unit cell relative to the lamellar basal plane obtained as the results of their investigation on PTT. They found that the twisting of lamellae is right-handed for the growth along the negative growth direction while it is left-handed for the positive growth direction. At the end of their discussion, despite the agreement with the Keith and Padden model, they stated that the value of 4° chain tilt did not appear to be sufficient for generation of the surface stresses required for twisted lamellar growth.
More recently, Rosenthal, et al. [
9] continued their research using nanobeam X-ray scattering and small angle X-ray scattering on PTT banded spherulites (from approximately 20 µm-thickness sample).
Figure 9 shows their proposed sketches of (a) the lamellar twist of PTT melt crystallized at 170 °C (BI) and 190 °C (BII) as well as (b) the hypothetical molecular model of PTT lamella formed at 170 and 190 °C. They found two temperature regions of BI (represented by 170 °C) and BII (represented by 190 °C) where banded spherulites are formed. They speculated that the chirality correlates with the growth axis polarity (i.e.,
a vs. −
a) and handedness of the lamellar helicoid (
L vs.
R) and can be conveniently expressed in terms of the chirality parameter pairs as illustrated in
Figure 9a. They also speculated that the inclination of the terminal segment of the crystalline stem protruding the lamellar surface is the key factor controlling the surface stresses (
Figure 9b).
X-rays data, diffraction as well as scattering, are indeed precise yet their data interpretations are tricky. Unlike microscopy techniques, X-ray techniques give patterns instead of magnified images. A periodic oscillation plot of diffracted X-ray intensity as a function of radial distance does not solely correspond to lamellar twisting. Branching of lamellae in two perpendicular arrangements of crystals, for example, could also be justified using the same data. Investigation of the interior lamellar arrangement using microscopy technique may become the further justification we need. PTT crystals in a 20–30 µm film sample may arrange in a concentric spherical shape (perhaps it is not a concentric cylindrical shape), meaning that the top surface measured radial distance of crystals with the same miller indices would vary for each layer according to the curvature of a sphere. The X-ray beam could penetrate through the whole sample thickness, and the diffraction pattern collected by the detector only shows an average result. How can one be so sure that lamellar twisting along the radial direction is the only true interpretation of such X-ray diffraction data? Perhaps the branching of lamellae in two perpendicular arrangements of crystals or other unprecedented proposed mechanisms may also be true for such oscillation intensity. Caution should be taken in interpreting such data.
In 2004, Chuang, et al. [
13] reported the link between microscopic structure and macroscopic morphology of PTT spherulites by probing the top surface profiles using AFM. They reported wavy-like concave (bright) and convex (dark) bands which correspond to trace-like lamellae and edge on lamellae, respectively, growing parallel to the radial direction.
Figure 10 shows the schematic model of hierarchic structure in the formation of PTT banded spherulite proposed by Chuang, et al. They suggested that the alternating arrangement of these edge-on and flat-on lamellae in PTT banded spherulites originated from two growth mechanisms based on intermittent growth rate. Fibrillation of the edge-on lamellae in forming the convex bands (ridges) slows down the growth rate; by contrast, development of the trace-like lamellae for forming the concave bands (valleys) accelerates the growth. The authors conjectured the existence of crystal/melt interface associated with oscillation dynamics driven by latent heat diffusion at the spherulite growth front. Such a phenomenon was considered as a common characteristic of complex space-time self-organization under a non-equilibrium crystallization condition.
The crystals underneath the top surface of a spherulite, nevertheless, can differ dramatically from those observed on the top surface. Without dissecting into the interior, lamellar assembly in a spherulite is incomplete. Thus, seeking the authenticity of the PTT banded spherulite, Woo et al. [
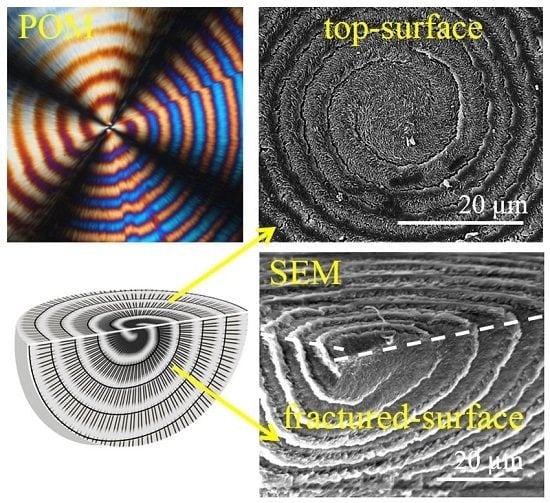
42] attempted to take an alternative approach from bulk dissection to perceive the behavior of interior PTT crystals by using scanning electron microscopy (SEM)—whether or not they continuously and monotonously twist during growth from a common center.
Figure 11 shows SEM images of virgin PTT-single-spiral spherulite melt-crystallized at 165 °C: (a) top surface and (b) top vs. fracture surface. Correlations between the top and fractured surfaces were obtained by tilting the fractured sample (thickness ca. 100 μm) to a certain angle, thus, the electron beam could hit both top and fractured surfaces at once. Wavy structures of lamellae present as alternating ridge–valley bands on the top surface are observed on the fractured surface. Instead of interpreting as lamellar twisting along the radial directions, Woo et al. [
42] found three-dimensional spiraling valley bands that actually separate the successive ridge bands in the interior of the PTT spherulites. These lamellae patterns pass through the top and fractured surfaces as indicated by white dashed lines in the graphs of
Figure 11.
6. Etching Effects on Distortion of Lamellae in PTT Banded Spherulites
Etching methods are long known to be effective in exposing the crystal assembly in contrast to amorphous domains in bulk polymer samples [
6,
7,
47,
48], although some chemical reaction during etching is sometimes inevitable [
48,
49]. Severe chemical changes or deformation of crystal shapes, however, must be cautioned against, as otherwise, interpretation of crystal morphology based on etched samples might be misleading. Woo, et al. [
48] reported with evidence that methylamine etching does not only induce physical degradation but also causes molecular reductions, chemical changes (transforms the ester linkages into amides), and also alters the original crystals shape of PTT spherulites. When dealing with semicrystalline polyester PTT with low degrees of crystallinity [
44,
45] and oxidation, potassium permanganate (KMnO
4) may serve as a good enchant in revealing the actual crystal assembly of PTT. For the purpose of the study, both chemical and physical etching will be briefly discussed.
6.1. Chemical Etching
Exposure to methylamine (MA) or methylamine-vapor was proved many years ago to be detrimental and degrading to chemical structures of many organic substances [
49]. Wang et al. [
47] even discussed evidence of twisting lamellae in poly(3-hydroxybutyric acid-
co-3-hydroxyvaleric acid) (PHBV) by methylamine-vapor etching on crystallized lamellae. Several other investigators also used methylamine as an etchant to expose the lamellae behind the polymer banded spherulites without considering the chemical reactivity of methylamine with the polymer itself. Li et al. [
6,
7] reported their results on methylamine-etched PTT banded spherulites (from the film surface observations) which show the periodic twist from protruded edge-on lamellar crystals to the flat-on lamellar crystals as they grow in the radial direction. By claiming that these findings were consistent with the results obtained by Ho et al. [
2] and Wang et al. [
3], Li et al. [
6,
7] supported the Keith and Padden model of unbalanced surface stresses induced lamellar twisting as the origin of banded spherulites in PTT.
Misinterpretation owing to methylamine-induced distortion of crystals might be a risk. However, in the past years and regardless of numerous investigations reported in the literature, there has been no direct evidence ever on lamellar twisting that perfectly matches the interval band spacing of the banded spherulites reported from the interior point of view. Thus, Woo, et al. [
42] adopted the methylamine etching method to expose the interior lamellar arrangements of banded spherulites. PTT bulk samples were exposed to methylamine vapor at ambient temperature for various etching periods from 2 to 24 h prior to microscopic characterization.
Figure 12 shows SEM micrographs of the top surface of PTT spherulites after various periods of MA-etching. Intermittent development of crevices started within 2–4 h; at 8 h of MA-exposure, crevices were significantly increased in number. Longer etching period up to 24h yields enlargement of the crevices dimension, although it does not significantly increase the number of crevices.
Spindle-like crystals of methylammonium salt observed on the unwashed sample are the by-product of methylamine reacted with the carbonyl carbons of the polyester, chain scissoring the polymer into the lower molecular weight of poly(trimethylene terephthalate)-amide. The methylammonium salt is water soluble and could be easily washed out by pure water. Thus, after being washed and dried (
Figure 12b–f), the PTT sample showed clearer geometry shape and curvature of crevices with better contrasts. Considering the dimensions of the crevices (1–2 μm) which are hundreds of times larger than the thickness of virgin PTT lamellae of approx. 8 nm as obtained by SAXS, it is clear that methylamine did not only wash out the inter-lamellar species but also induced lamellae distortion in terms of twisting or inter-bundle cracking into micrometer-sized crevices.
SEM images of the interior architecture of 16h-MA etched PTT banded spherulites fractured in several different angles are shown in
Figure 13. For a typical concentric-banded spherulite (
Figure 13a,b), the ridge and valley on the top surface are reversely connected to those on the fractured surface (i.e., the ridge of the top surface to the valley of the fractured surface, and vice versa). The band spacing is varying, corresponding to the nucleus position. When the nucleus is on top, the band spacing on top, the fractured surfaces are perfectly matched (8 µm); when it is buried under, the band spacing on the top is larger than that on the fractured surface. Along the peripheral surface (
Figure 13c), impingement with other spherulites occurs, taking a spherical shape; in addition, the crystal lamellae beneath the ridges are stacked along the longitude (squared in red). By contrast, under the valley, the lamellae are stacked together along its latitude (squared in yellow). Truncated banded structures are observed on the peripheral surface, which indicates a periodic development of each band. Similar phenomena could also be observed from the internal point of view (
Figure 13d). For spiral-banded spherulite, the ridge and valley on the top surface are correspondingly interconnected to those on the fractured surface. Underneath the top surface, instead of radially twisted, the lamellae were found circumferentially spiraling (as marked with white dashed lines in
Figure 13e) which further strengthen the discrete development of lamellae in the radial direction. Lamellae at the successive ridges are interconnected by other lamellae at the valley (shell). The development of lamellae at the ridges might also be initiated, restricted, and/or terminated due to the existence of this shell. The lamellae may also be slightly bent/curved with respect to the spiral direction to fill the space of each band discretely. A scheme (
Figure 13f) was constructed to illustrate the 3D spiral development of the spherulite as discussed above.
The inter-lamellae bundle crevices are localized always in the radial direction (i.e., perpendicular to the circular/spiral direction) on either the cross-sectional or top surfaces. The directional phenomenon indicated that the radially-oriented lamellae in the banded PTT spherulites, either on the top surface or interiors, are periodically disrupted by lamellae of a different orientation. Immediately as the interior tangential lamellae emerge from the interior to the top surface, they bend outward and toward the radial direction. These protruded lamellae from the interior to the top surface may bend and twist irregularly as they emerge from the interior. These twisted lamellae on the top surface of spherulites thus cannot be considered as evidence of continuous lamellar twisting, as they are not monotonously twisting up and down like single crystals, only on the top surface. These irregularly twist lamellae actually were a partial continuation from the interior tangential crystal bundles that bend 90° toward the radial direction as they emerge to the top surface of the spherulites.
6.2. Alternative Etching with None or Less Lamellae-Distortion
Owing to concerns on methylamine etching that causes not only chemical changes but also lamellae shape distortion, alternative etching methods were attempted to better preserve the lamellae geometry while exposing the lamellae structures in banded PTT spherulites. Twenty minutes etching period using two percent weight KMnO
4 in acid conditions [1:1 H
2SO
4:H
3PO
4] was found favorable for enhancing the crystal contrast of PTT spherulites. Prolonging the etching period to one hour causes severe damage on the crystals, enlarging the crevice between bands, and inducing separation of the bands. POM and SEM images of the top surface of 20 min-KMnO
4-etched PTT spherulites reproduced from [
42] are shown in
Figure 14. The morphology of KMnO
4-etched PTT banded spherulites differ from that of MA-etched PTT discussed earlier, although they do express the same message. Instead of inter-lamellae bundle crevices, circumferential crevices were found between the successive bands (circular dark regions in POM
Figure 14a). Curved lamellae exposed at higher magnification (
Figure 14d) on the top surface after KMnO
4 etching correspond to the curved lamellae bundle on the MA-etched PTT sample, while the circular crevices correspond to the shell observed on the MA-etched PTT sample.
The discontinuities of spherulite growth in radial directions were further strengthened by the SEM images of the top vs. fractured surface of KMnO
4-etched PTT spherulites shown in
Figure 15. Both fractured and top surfaces of PTT clearly show the periodic manner of crystal lamellae deposition along radial directions. Color contrast along radial directions in the SEM images indicates the uneven crystal density distributions and/or contrast orientations. KMnO
4-etched concentric-banded spherulite (
Figure 15a) shows discrete bands resembling the onion bulbs, separated by the circular crevices. Single- (
Figure 15b) and double-spiral banded spherulites (
Figure 15c) show the 3-D spiral arm(s) wrapping one another from the inner core to the outer edge and separated by narrow crevice(s). Each arm is composed of thicker crystal lamellae (appear brighter) that branch and/or tapper into finer crystal fibrils (appear darker) as they adjoin each other. The circular/spiral crevice(s) are the interconnecting layer(s) of adjoined crystal fibrils between the successive bands as illustrated in the scheme.
Woo, et al. [
41] produced three schemes of progressive growth of banded PTT spherulites as a summary of their observations of thin film samples. Concentric banded spherulites (type-1) has a dot-like nucleus (with a sheaf-like structure), while both spiral spherulites (type-2 and type-3) have an S-shape initial crystal structure. During the initial growth, the 3-D spiral rotation might be induced by the unbalanced nucleus position or compactness of space requirement, which resulted from the nonequilibrium to equilibrium process of nucleation. Depending on the surface topographical slope, one of the arms may grow faster and wrap-up the other arm then continue overwrapping itself as it grows further (type-2) or both arms may grow to overwrap each other (type-3). As mention earlier in the text, the growing arm(s) consist of thicker crystal lamellae that are initially aligned with their long axes parallel to the spiral direction and gradually align perpendicular to the spiral direction (i.e., in the radial direction) as they grow further from the core. To achieve maximum coverage, these crystal lamellae (brighter area) branch and/or tapper into finer crystal fibrils (bright gray area around the thicker crystal). As the successive bands adjoin one to another, these fibrils are found to bend in the circular/spiral direction.
Based on Woo’s work in observing the interior lamellar assembly of banded spherulites [
42], there are several highlighted results that are worth consideration. The unetched single-spiral banded spherulite has one of its arms wrapping the other arm and grows as 3-D spiral convex bands of crystal lamellae separated by concave bands of fibril lamellae. The MA-etched concentric banded spherulite has its valleys and ridges on the top in opposition to the valleys and ridges on the fractured surface. Lamellae on the ridges (on both top and fractured surfaces) are arranged in radial directions and separated by crevices. Intermittent development of crevices indicates the periodically disrupted growth of lamellae of the radial direction by lamellae of circumferential orientation. KMnO
4-etched double-spiral banded spherulite has both arms overwrapping each other in a spiral manner. The two growing arms are separated by spiral crevices. Each arm consists of thicker crystal lamellae that branch and/or taper into finer crystal fibrils to fill the space before they collide to the crystal of another arm, allowing maximum coverage of the spherulite. Thus, instead of the conventional model of continuously spiraling giant lamellae, the ring-banded PTT spherulite supposedly is composed of polycrystals, oriented perpendicularly and layered periodically, branching out and colliding among the neighboring bands.