New Iron(II) Spin Crossover Complexes with Unique Supramolecular Networks Assembled by Hydrogen Bonding and Intermetallic Bonding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Preparation of Compound 1a
2.2.2. Preparation of Compound 1b
2.3. X-ray Crystallography
2.4. Magnetic Measurements
3. Results and Discussion
3.1. Crystal Structures
3.1.1. Crystal Structure of Compound 1a (T = 298 K)
3.1.2. Crystal Structure of Compound 1b (T = 298 K)
3.1.3. Structure of Compound 1b (T = 90 K)
3.2. Thermal Analysis
3.3. Magnetic Properties
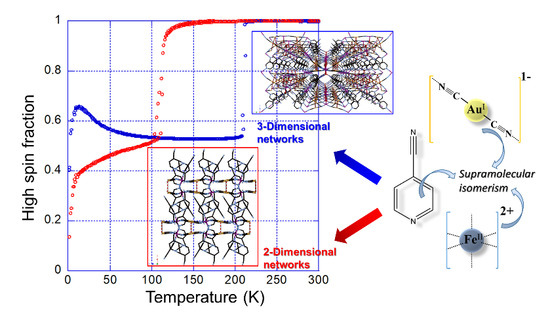
3.3.1. Thermal Dependence Magnetic Behavior of Compound 1a
3.3.2. Thermal Dependence Magnetic Behavior of Compound 1b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Real, J.A.; Andrés, E.; Munoz, M.C.; Julve, M.; Granier, T.; Bousseksou, A.; Varret, F. Spin crossover in a catenane supramolecular system. Science 1995, 268, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Miyamachi, T.; Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Joly, L.; Scheurer, F.; Rogez, G.; Yamada, T.K.; Ohresser, P.; et al. Robust spin crossover and memristance across a single molecule. Nat. Commun. 2012, 3, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentili, D.; Givaja, G.; Mas-Ballesté, R.; Azani, M.-R.; Shehu, A.; Leonardi, F.; Mateo-Martí, E.; Greco, P.; Zamora, F.; Cavallini, M. Patterned conductive nanostructures from reversible self-assembly of 1D coordination polymer. Chem. Sci. 2012, 3, 2047–2051. [Google Scholar] [CrossRef]
- Gentili, D.; Demitri, N.; Schäfer, B.; Liscio, F.; Bergenti, I.; Ruani, G.; Ruben, M.; Cavallini, M. Multi-modal sensing in spin crossover compounds. J. Mater. Chem. C 2015, 3, 7836–7844. [Google Scholar] [CrossRef]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(ii) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Chiruta, D.; Jureschi, C.-M.; Linares, J.; Garcia, Y.; Rotaru, A. Lattice architecture effect on the cooperativity of spin transition coordination polymers. J. Appl. Phys. 2014, 115, 053523. [Google Scholar] [CrossRef] [Green Version]
- Kitazawa, T.; Gomi, Y.; Takahashi, M.; Takeda, M.; Enomoto, M.; Miyazaki, A.; Enoki, T. Spin-crossover behaviour of the coordination polymer FeII(C5H5N)2NiII(CN)4. J. Mater. Chem. 1996, 6, 119–121. [Google Scholar] [CrossRef]
- Galet, A.; Muñoz, M.C.; Martinez, V.; Real, J.A. Supramolecular isomerism in spin crossover networks with aurophilic interactions. Chem. Commun. 2004, 2268–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, M.C.; Gaspar, A.B.; Galet, A.; Real, J.A. Spin-Crossover Behavior in Cyanide-Bridged Iron(II)-Silver(I) Bimetallic 2D Hofmann-like Metal-Organic Frameworks. Inorg. Chem. 2007, 46, 8182–8192. [Google Scholar] [CrossRef] [PubMed]
- Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A. Spin-Crossover Behavior in Cyanide-bridged Iron(II)−Gold(I) Bimetallic 2D Hofmann-like Metal−Organic Frameworks. Inorg. Chem. 2008, 47, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Kosone, T.; Kachi-Terajima, C.; Kanadani, C.; Saito, T.; Kitazawa, T. A two-step and hysteretic spin-crossover transition in new cyano-bridged hetero-metal FeIIAuI 2-dimensional assemblage. Chem. Lett. 2008, 37, 422–423. [Google Scholar] [CrossRef]
- Kosone, T.; Kanadani, C.; Saito, T.; Kitazawa, T. Synthesis, crystal structures, magnetic properties and fluorescent emissions of two-dimensional bimetallic coordination frameworks FeII(3-fluoropyridine)2[AuI(CN)2]2 and MnII(3-fluoropyridine)2[AuI(CN)2]2. Polyhedron 2009, 28, 1930–1934. [Google Scholar] [CrossRef]
- Kosone, T.; Kanadani, C.; Saito, T.; Kitazawa, T. Spin crossover behavior in two-dimensional bimetallic coordination polymer FeII(3-bromo-4-picoline)2[AuI(CN)2]2: Synthesis, crystal structures, and magnetic properties. Polyhedron 2009, 28, 1991–1995. [Google Scholar] [CrossRef]
- Kosone, T.; Tomori, I.; Kanadani, C.; Saito, T.; Mochida, T.; Kitazawa, T. Unprecedented three-step spin-crossover transition in new 2-dimensional coordination polymer {FeII(4-methylpyridine)2[AuI(CN)2]2}. Dalton Trans. 2010, 39, 1719–1721. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, J.; Ueno, S.; Kawasaki, T.; Kitazawa, T. Ligand 4-X pyridine (X=Cl, Br, I) dependence in Hofmann-type spin crossover complexes: Fe(4-Xpyridine)2[Au(CN)2]2. Inorg. Chim. Acta 2016, 445, 17–21. [Google Scholar] [CrossRef]
- Kosone, T.; Kawasaki, T.; Tomori, I.; Okabayashi, J.; Kitazawa, T. Modification of Cooperativity and Critical Temperatures on a Hofmann-Like Template Structure by Modular Substituent. Inorganics 2017, 5, 55. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction for Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXL, Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Kosone, T.; Kitazawa, T. Guest-dependent spin transition with long range intermediate state for 2-dimensional Hofmann-like coordination polymer. Inorg. Chim. Acta 2016, 439, 159–163. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kachi-Terajima, C.; Saito, T.; Kitazawa, T. Triply Interpenetrated Structure of {MnII(L)2[AgI(CN)2]2}{MnII(H2O)2[AgI(CN)2]2} (L = 4-CNpy or py-4-aldoxime). Bull. Chem. Soc. Jpn. 2008, 81, 268–273. [Google Scholar] [CrossRef]



| 1a (150 K) | 1b (298 K) | 1b (90 K) | |
|---|---|---|---|
| Empirical formula | C19H8Au2FeN9 | C20H11Au4Fe2N12O2 | C20H11Au4Fe2N12O2 |
| Formula weight | 812.13 | 1350.97 | 1350.97 |
| Crystal size/mm3 | 0.55 × 0.28 × 0.22 | 0.15 × 0.13 × 0.05 | 0.38 × 0.15 × 0.05 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic |
| a/Å | 21.658(2) | 7.6273(7) | 7.6243(4) |
| b/Å | 13.7647(15) | 30.007(3) | 29.1960(16) |
| c/Å | 15.5895(17) | 13.7694(13) | 13.5000(7) |
| V/Å3 | 4647.4(9) | 3151.5(5) | 3005.1(3) |
| Space group | Pbcn | Pnma | Pnma |
| Z value | 8 | 4 | 4 |
| Dcalc | 2.321 | 2.847 | 2.986 |
| F(000) | 2952 | 2396 | 2396 |
| No. of reflections | 28,466 | 19,996 | 20,977 |
| No. of observations | 5443 | 3976 | 3808 |
| Parameters | 284 | 196 | 186 |
| Temperature/K | 150(2) | 298 | 90 |
| Final R1, Rw (I > 2s) | 0.0411, 0.1043 | 0.0474, 0.1168 | 0.0396, 0.1048 |
| Final R1, Rw (all data) | 0.0715, 0.1197 | 0.0730, 0.1346 | 0.0439, 0.1212 |
| Goodness-of-fit | 1.050 | 0.965 | 1.413 |
| Bond Lengths (Å) for 1a (150 K) | Bond Angles (°) for 1a (150 K) | |
|---|---|---|
| Fe(1)–N(1): 2.234(9) | N(1)–Fe(1)–N(3): 175.9(3) | N(5)–Fe(1)–N(8): 90.0(3) |
| Fe(1)–N(3): 2.227(8) | N(1)–Fe(1)–N(5): 93.0(3) | N(6)–Fe(1)–N(7): 88.2(3) |
| Fe(1)–N(5): 2.161(8) | N(1)–Fe(1)–N(6): 94.5(3) | N(6)–Fe(1)–N(8): 176.4(3) |
| Fe(1)–N(6): 2.129(8) | N(1)–Fe(1)–N(7): 89.4(3) | N(7)–Fe(1)–N(8): 95.1(3) |
| Fe(1)–N(7): 2.121(8) | N(1)–Fe(1)–N(8): 84.0(3) | C(13)–N(5)–Fe(1): 167.4(8) |
| Fe(1)–N(8): 2.146(8) | N(3)–Fe(1)–N(5): 86.9(3) | C(14)–N(6)–Fe(1): 162.4(8) |
| Au(1)–C(13): 1.999(9) | N(3)–Fe(1)–N(6): 89.6(3) | C(15)–N(7)–Fe(1): 163.2(8) |
| Au(1)–C(16): 1.990(9) | N(3)–Fe(1)–N(7): 91.1(3) | C(16)–N(8)–Fe(1): 161.3(9) |
| Au(2)–C(14): 1.986(9) | N(3)–Fe(1)–N(8): 91.9(3) | C(13)–Au(1)–C(16): 178.0(4) |
| Au(2)–C(15): 1.981(10) | N(5)–Fe(1)–N(6): 86.8(3) | C(14)–Au(2)–C(15): 176.8(4) |
| N(5)–Fe(1)–N(7): 174.6(3) | ||
| Bond Lengths (Å) for 1b (298 K) | Bond Angles (°) for 1b (298 K) | |
| Fe(1)–N(1): 2.226(7) | N(1)–Fe(1)–N(1): 180.0(3) | O(1)–Fe(2)–O(2): 169.5(6) |
| Fe(1)–N(3): 2.157(7) | N(1)–Fe(1)–N(3): 90.0(3) | O(1)–Fe(2)–N(5): 96.6(4) |
| Fe(1)–N(4): 2.144(7) | N(1)–Fe(1)–N(4): 89.5(3) | O(1)–Fe(2)–N(6): 89.9(3) |
| Fe(2)–O(1): 2.078(13) | N(3)–Fe(1)–N(3): 180.0(3) | O(2)–Fe(2)–N(5): 90.8(4) |
| Fe(2)–O(2): 2.158(9) | N(3)–Fe(1)–N(4): 91.3(3) | O(2)–Fe(2)–N(6): 83.3(3) |
| Fe(2)–N(5): 2.130(8) | N(4)–Fe(1)–N(4): 180.0(4) | N(5)–Fe(2)–N(5):89.5(5) |
| Fe(2)–N(6): 2.187(8) | C(7)–N(3)–Fe(1): 167.1(8) | N(5)–Fe(2)–N(6): 85.6(3) |
| Au(1)–C(8): 1.985(9) | C(8)–N(4)–Fe(1): 166.6(8) | N(6)–Fe(2)–N(6): 98.7(4) |
| Au(1)–C(9): 1.978(9) | C(8)–Au(1)–C(9): 175.9(4) | N(5)–Fe(2)–N(6): 172.3(3) |
| Au(2)–C(7): 1.992(9) | C(7)–Au(2)–C(10): 176.6(3) | C(9)–N(5)–Fe(2): 169.6(8) |
| Au(2)–C(10): 1.992(9) | C(10)–N(6)–Fe(2): 162.0(8) | |
| Bond Lengths (Å) for 1b (90 K) | Bond Angles (°) for 1b (90 K) | |
| Fe(1)–N(1): 1.998(7) | N(1)–Fe(1)–N(1): 180.0(4) | O(1)–Fe(2)–O(2): 169.1(4) |
| Fe(1)–N(3): 1.937(7) | N(1)–Fe(1)–N(3): 90.5(3) | O(1)–Fe(2)–N(5): 96.2(3) |
| Fe(1)–N(4): 1.941(7) | N(1)–Fe(1)–N(4): 90.4(3) | O(1)–Fe(2)–N(6): 89.3(3) |
| Fe(2)–O(1): 2.095(9) | N(3)–Fe(1)–N(3): 180.0(2) | O(2)–Fe(2)–N(5): 91.5(3) |
| Fe(2)–O(2): 2.112(9) | N(3)–Fe(1)–N(4): 90.3(3) | O(2)–Fe(2)–N(6): 83.6(3) |
| Fe(2)–N(5): 2.132(7) | N(4)–Fe(1)–N(4): 180.0(4) | N(5)–Fe(2)–N(5): 90.6(4) |
| Fe(2)–N(6): 2.190(8) | C(7)–N(3)–Fe(1): 170.7(7) | N(5)–Fe(2)–N(6): 85.0(3) |
| Au(1)–C(8): 1.985(9) | C(8)–N(4)–Fe(1): 172.3(7) | N(6)–Fe(2)–N(6): 98.9(4) |
| Au(1)–C(9): 1.977(9) | C(8)–Au(1)–C(9): 174.8(3) | N(5)–Fe(2)–N(6): 173.4(3) |
| Au(2)–C(7): 1.983(9) | C(7)–Au(2)–C(10): 174.2(3) | C(9)–N(5)–Fe(2): 168.6(7) |
| Au(2)–C(10): 1.989(8) | C(10)–N(6)–Fe(2): 161.7(7) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosone, T.; Tomori, I.; Akahoshi, D.; Saito, T.; Kitazawa, T. New Iron(II) Spin Crossover Complexes with Unique Supramolecular Networks Assembled by Hydrogen Bonding and Intermetallic Bonding. Crystals 2018, 8, 415. https://doi.org/10.3390/cryst8110415
Kosone T, Tomori I, Akahoshi D, Saito T, Kitazawa T. New Iron(II) Spin Crossover Complexes with Unique Supramolecular Networks Assembled by Hydrogen Bonding and Intermetallic Bonding. Crystals. 2018; 8(11):415. https://doi.org/10.3390/cryst8110415
Chicago/Turabian StyleKosone, Takashi, Itaru Tomori, Daisuke Akahoshi, Toshiaki Saito, and Takafumi Kitazawa. 2018. "New Iron(II) Spin Crossover Complexes with Unique Supramolecular Networks Assembled by Hydrogen Bonding and Intermetallic Bonding" Crystals 8, no. 11: 415. https://doi.org/10.3390/cryst8110415
APA StyleKosone, T., Tomori, I., Akahoshi, D., Saito, T., & Kitazawa, T. (2018). New Iron(II) Spin Crossover Complexes with Unique Supramolecular Networks Assembled by Hydrogen Bonding and Intermetallic Bonding. Crystals, 8(11), 415. https://doi.org/10.3390/cryst8110415