1. Introduction
Barium tungstate (BaWO
4) crystals are an interesting and relatively new medium for stimulated Raman scattering for applications in Raman shifters of laser radiation [
1]. BaWO
4 has the largest energy gap among several tungstates with a scheelite structure, space group
I4
1/
a, equal to 5.26 eV [
2]. Barium tungstate is a widely investigated inorganic optical material due to its attractive emission properties. The properties strongly depend on the crystal structure and morphology. The same dependence shows the photocatalytic properties of the crystal [
3].
The Ce
3+-doped and undoped samples of alkali earth metal tungstates MWO
4 (M=Ca, Sr, and Ba) microcrystalline phosphors were synthesized by a co-precipitation method in a controlled pH environment by Double et al. [
3]. X-Ray Diffraction (XRD) pattern and Scanning Electron Microscopy (SEM) micrographs revealed the formation of the desired tetragonal phase of the scheelite-type structure with the grain size of a few micrometers. Phosphor excited by 280 nm showed a broad emission band in the visible region (400–650 nm), with a peak in the blue region at around 465 nm, which is characteristic of the (WO
4)
2− complex. More extended investigations and quantum mechanical calculations from the morphology point of view were performed for BaWO
4 crystals obtained using the same technique by Oliveira et al. [
4]. Liu et al. explored the growth mechanism leading to the observed range of morphologies through in situ Transmission Electron Microscopy (TEM) and in situ Atomic Force Microscopy (AFM) [
5]. Ke et al. have provided an integrated overview of the fundamentals and recent progress of MWO
4-based photocatalysts [
6]. Oliveira et al. also investigated the photoluminescence spectrum in BaWO
4 crystals centered at about 522 nm. They assigned the emission to the charge transfer in the [MO
4]
2− (M = Mo and W) complex [
7]. Yellow emission was reported from BaWO
4: Sm
3+ phosphors by Shi et al. [
8].
Good quality BaWO
4 crystals can be grown by the Czochralski technique and doped with rare-earth ions [
9,
10,
11]. We have grown pure BaWO
4, as well as BaWO
4 doped with 0.5 at. % Ce and 1 at. % Ce, single crystals using the above technique. The absorption and emission spectra of the crystals were investigated by Włodarczyk et al. in [
12]. In the UV, the crystals showed typical absorption for Ce
3+, with two bands that peaked at 320 nm and 285 nm, being associated with lowest energy 4f-5d transitions. However, only very weak Ce
3+ luminescence was observed, even at 10 K, contrary to the above-mentioned report on this subject [
3].
Doping with trivalent ions requires charge compensation which may be provided, for example, by structural defects or proper co-doping with alkaline metal ions. Voronina et al. obtained BaWO
4: Nd
3+ single crystals by the Czochralski method using compensating dopants: Nb
5+ and Na
+ [
13]. Therefore, we decided to co-dope the crystals with Na ions. In this paper, we analyze local symmetries of cerium ions in BaWO
4:0.5 at. % Ce; BaWO
4:1 at. % Ce; BaWO
4:0.5 at. % Ce, 1 at. % Na; and BaWO
4:1 at. % Ce, 2 at. % Na single crystals. Radioluminescence spectra and light yield measurements of the above crystals and pure BaWO
4 are also presented and discussed for comparison.
2. Materials and Methods
Single crystals of BaWO
4; BaWO
4:0.5 at. % Ce; BaWO
4:1 at. % Ce; BaWO
4: 0.5 at. % Ce, 1 at. % Na; and BaWO
4:1 at. % Ce, 2 at. % Na were grown from an inductively heated iridium crucible by the Czochralski method on a Malvern MSR4 puller. The reagents—BaCO
3 (5N), WO
3 (4N), CeO
2 (4N), and Na
2CO
3(5N) (Sigma – Aldrich, Poznań, Poland r Fluka, Poznań, Poland)—were dried in 300 °C for 12 h before weighing and mixing them in stoichiometric molar ratios. Starting materials were pressed into cylindrical pellets and then put into the crucible, which was 40 mm in diameter. BaWO
4 single crystals were grown on an iridium rod used as a seed with a pulling rate of 3 mm/h and a speed of rotation of 20 rpm in ambient (N
2) atmosphere. Obtained crystal boules of a 20 mm diameter and 40 mm length were colorless and transparent (
Figure 1). BaWO
4: Ce crystals co-doped with Na show some cracks.
Phase analysis and structural refinement were performed on powdered single crystals by X-ray powder diffraction using Ni-filtered Cu-Kα radiation with a Siemens D5000 diffractometer (Siemens, München, Germany). Data were collected in the range 10° < 2Θ < 110°, with a step o 0.02° and an averaging time of 10 s/step. The powder diffraction patterns were analyzed by the Rietveld refinement method using the DBWS-9807 program.
The structure of the BaWO
4 single crystal is shown in
Figure 2. From the local symmetry point of view, among three planes distinctly different seems to be an ab-plane. The space group is I4
1/a and symmetry C
64h, in which Ba atoms are coordinated to eight O atoms, while W atoms exhibit a tetragonal coordination of O atoms; thus, the building blocks of the BaWO
4 crystal are deltahedral [BaO
8] and tetrahedral [WO
4] clusters [
14].
The EPR spectroscopic measurements were carried out using a standard X-band Bruker E-500 EPR spectrometer (Karlsruhe, Germany) with the magnetic induction range 0–1.4 T, the micro field modulation of 100 kHz, and the temperature range (3–300) K.
The magnetic induction was scaled with an NMR magnetometer. Measurements were performed under the flow of helium gas, using an Oxford Instruments (Abingdon, Oxfordshire, England) flowing liquid He cryostat to control the temperature. The samples used for EPR purposes have been shaped as 2.5 × 2.5 × 3.5 mm
3 parallelepipeds with planes perpendicular to crystallographic axes. In order to determine the local symmetry and localization of the paramagnetic probe in the crystal lattice, the angular dependences of Ce
3+ have been drawn (Figures 5, 7 and 8). The laboratory axis system (LAS;
a, b, c) corresponded to the crystallographic axis system (CAS;
x,
y,
z). Axes
a,
b, and
c were perpendicular to the magnetic induction
B. The EPR-NMR program was used to find spin-Hamiltonian parameters [
15]. The
g-factors were obtained by the complete diagonalization of the spin Hamiltonian and least-squares fitting to the observed spectra.
Radioluminescence spectra at various temperatures between 10 and 350 K were recorded with a custom-made set-up consisting of an Inel X-ray generator with a Cu-anode tube (Artenay, Loiret, France), an ARC SP-500i monochromator (Acton, MA, USA), a Hamamatsu R928 photomultiplier tube (Hamamatsu City, Shizuoka, Japan), and an APD Cryogenics closed-cycle helium cooler (Allentown, PA, USA) controlled with a Lake Shore 330 unit (Westerville, OH, USA). Pulse height spectra were measured at room temperature (RT) under gamma excitation from a 137Cs source (662 keV). The dedicated set-up consisted of a Hamamatsu R2059 photomultiplier tube (Hamamatsu City, Shizuoka, Japan), a Canberra 2005 integrating preamplifier (Meriden, CT, USA), a Canberra 2022 spectroscopy (Meriden, CT, USA) amplifier (with shaping time adjusted at 12 µs), and a Tukan 8 k USB multichannel analyzer (Świerk, Poland).
3. X-ray Measurements
The X-ray diffraction patterns for all the crystals investigated are presented in
Figure 3. The measurements were performed at room temperature. The diffraction peak positions and intensities of all of the investigated crystals are consistent with the Joint Committee on Powder Diffraction Standards (JCPDS) Card No: 43-0646 of BaWO
4. The structure of the crystals is tetragonal and belongs to the I4
1/a space group. Lattice parameters have been determined as
a = 5.6149(1) Å,
c = 12.7201(2) Å for BaWO
4: 0.5 at. % Ce;
a = 5.6152(1) Å,
c = 12.7209(3) Å for BaWO
4: 1 at. % Ce;
a = 5.6180(1) Å,
c = 12.7141(2) Å for BaWO
4: 0.5 at. % Ce, 1 at. % Na; and
a = 5.619(1) Å,
c = 12.717(2) Å for BaWO
4: 1 at. % Ce, 2 at. % Na. The pure BaWO
4 has a tetragonal structure with unit cell dimensions of a = 5.61 Å, c = 12.71 Å [
16]. Rocking curves for the symmetrical (400) reflections have shown the following values of FWHM: 0.047°, 0.052°, 0.108°, and 0.126° for BaWO
4: 0.5 at. % Ce; BaWO
4: 1 at. % Ce; BaWO
4: 0.5 at. % Ce, 1 at % Na; and BaWO
4: 1 at. %Ce, 2 at % Na, respectively. This indicates a good enough quality of the crystals.
Ce3+ ions are only expected to substitute for Ba2+ ions in the BaWO4 structure owing to the respective ionic radii of cations (Ce3+ − R = 1.143 Å, Ba2+ − R = 1.142 Å, W6+ − R = 0.74 Å, Na+ − R = 1.18 Å). In the BaWO4 crystal, barium ions have four different crystallographic positions.
4. EPR Results
Due to the complexity of the obtained EPR results (many high and low symmetry centers), we only concentrate on the influence of sodium co-doping on crystal quality. The results of EPR studies of BaWO
4: Ce crystals and crystals co-doped with Na for several temperatures are presented in
Figure 4.
The EPR signal consists of one intense (main) EPR line and four or more weaker EPR lines assigned to cerium ions at different symmetry sites. The latter appear due to the substituting of some cerium ions at interstitial positions in view of different valences between barium (2+) and cerium (3+) ions (vacant compensation) and the presence of sodium co-dopant (vacant and co-dopant compensation). They all vanish over 60 K, as is usual for rare-earth ions. As cerium has no isotope with non-zero nuclear spin, the EPR spectrum of Ce3+ in a single crystallographic site is usually composed of a single line. The free cerium (Ce3+) ion has a 4f1 electronic configuration. A low symmetry of Ce3+ sites in the investigated crystals splits the six-fold degenerated 2F5/2 ground state into three degenerate levels, referred to as Kramers doublets. Only the lowest doublet is populated at liquid helium temperature, so the cerium ions system can be described by a fictitious spin S = 1/2. Thus, cerium is a Kramers ion with S = 1/2, L = 3, and J = 5/2, where S, L, and J are the spin, orbital, and total momentum, respectively.
The crystals co-doped with sodium seem to have a higher real concentration of cerium ions than those which are single doped. The same conclusions can be derived for the other two investigated crystals. Therefore, we assigned the following spin-Hamiltonian to analyze the local symmetry of cerium ions:
In
Figure 5, the roadmap of the main center in all planes:
ab,
bc, and
ca for BaWO
4: 0.5 at. % Ce crystal is presented. From the fitting of Equation (1) using the EPR-NMR program, the following parameters of
g-matrix for the center can be assigned to g
x = 1.506(1), g
y = 1.506(1), and g
z = 2.712(2) (see
Table 1).
Temperature dependence of the EPR line has shown ferromagnetic-like interactions between cerium ions, with Curie-Weiss temperature, Θ ~ 1 K. The linewidth vs. temperature revealed an increasing exponential tendency with increasing temperature. It shows one phonon at the lower temperatures and a Raman + Orbach effect (
Figure 6) at the higher temperatures, according to the equation B = B
o + Aexp(−W/kT), where B
o = 1.12 mT, A = 1390 mT, and W/k = 173 K. Exponential change of the B could be connected with the spin-lattice relaxation processes involving excited states of Ce
3+ ions.
Besides the central line in the EPR spectrum, corresponding to high symmetry cerium ions (
C4), other lines of lower symmetry are observed in the spectrum. They are seen in
Figure 7, where angular dependencies along the three directions
a,
b, and
c for BaWO
4: 0.5 at. % Ce single crystal are presented.
Axes
a,
b, and
c are perpendicular to the magnetic field induction,
B. The squares are experimental points and the solid lines are the fitting of the spin Hamiltonian parameters to the paramagnetic center. From
Figure 7a–c, one can find that, besides one line of high symmetry (solid line No. 1), there are at least two low symmetry centers. To each of the centers, two lines are assigned due to low symmetry. The symmetry of the centers seems to be
C2. Such a specific structure of the EPR signal, composed of two sets of doublets, indicates the existence of two magnetically inequivalent positions of Ce
3+ ions for each site. As one can see from
Figure 7d, EPR lines do not maintain continuity during a transition from the
c axis to the
b axis. This means that the laboratory
c axis does not correspond to the crystallographic
z axis. For monoclinic symmetry, this is obvious (γ ≠ 90 deg). The tilt of axis
c is as high as 10 and 11 degrees for lines 3 (3a and 3b,
Figure 7a) and 2 (2a and 2b,
Figure 7a), respectively. This confirms the
C2 symmetry of the cerium ions. The same conclusion can be derived from the EPR spectra measured for the BaWO
4 single crystal doping with 1% cerium ions. An entire other conclusion can be derived from the spectra of the BaWO
4: Ce crystals co-doped with sodium ions.
In
Table 1, spin-Hamiltonian parameters, tilt, and the kind of symmetry of the cerium centers are gathered.
In
Figure 8, angular dependencies are compared for BaWO
4: 0.5 at. % Ce and BaWO
4: 1 at. % Ce, 2 at. % Na single crystals, respectively. In the BaWO
4: Ce crystals doped with sodium ions, one can distinguish a single line assigned to cerium ions at local symmetry
C4, two doublets assigned to cerium ions at local symmetry
C2, and one doublet responsible for cerium ions at local
C2v symmetry. As one can see, EPR lines assigned to low symmetry centers are more symmetric in the case of Na co-doping. This represents a clearer structure of the Na co-doped crystal. It explains the higher real concentration of cerium ions in BaWO
4:Ce single crystals co-doped with Na. So, co-doping with Na leads to the more perfect crystal structure of BaWO
4:Ce single crystals and allows a higher real concentration of cerium ions to be introduced. However, it still does not allow photoluminescence (PL) originating from Ce
3+ ions to be obtained. Spin-Hamiltonian parameters, tilt, and the kind of cerium ions local symmetry for all of the investigated crystals are gathered in
Table 1. A more detailed analysis of the EPR properties of BaWO
4 single crystals doped with cerium ions is presented in [
17].
5. Radioluminescence Measurements
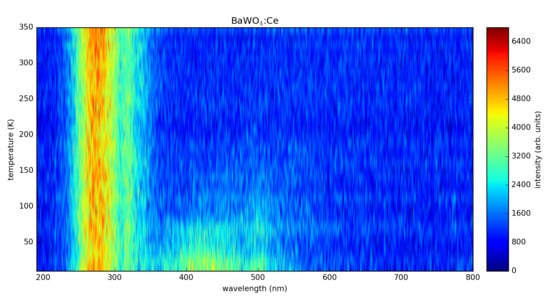
In
Figure 9, radioluminescence spectra at selected temperatures from the range (10–350) K are presented for the more representative cases: pure BaWO
4 and BaWO
4: 0.5 at. % Ce.
In both cases, two spectral regions can be distinguished. A double band peaking at about 280 and 310 nm is thermally very stable, maintaining the same intensity between 10 and 350 K (against any impression, the spectra have not been normalized). On the contrary, the other band, also with a double structure (peaks at about 410–430 and 500 nm), is strongly quenched with increasing temperature, almost vanishing above RT. Although there are some minor differences, mainly in the case of a longer wavelength band, we suppose that all the emissions are related to the BaWO
4 host. Since there are no premises of the Ce
3+ 5d-4f emission, it seems that either the energy transfer from the BaWO
4 host is extremely weak or the 5d levels of Ce
3+ emerge in the conduction band. We note that Cavalcante et al. have investigated the photoluminescence mechanism of BaWO
4 micro-powders and have found that the PL emission is caused by the structural defects and/or distortions on the [WO
4] tetrahedron groups by the exciting radiation [
18]. A strong emission band at about 280 nm was not recorded even by Dabre et al. [
3], who analyzed the photoluminescence properties of microcrystalline BaWO
4: Ce powders. They have found that PL excitation spectra exhibit a broad band in the UV region, with a peak at 280 nm, and the emission spectrum shows a broad band in the visible region, with a peak in the blue region (400–650 nm) [
3]. It seems that this kind of photoluminescence may be assigned to excitonic luminescence.
7. Discussion and Conclusions
The good quality of the BaWO
4:Ce
3+ 0.5 at. %; BaWO
4:Ce
3+ 0.5 at. %, Na
+ 1 at. %; BaWO
4:Ce
3+ 1 at. %; and BaWO
4: Ce
3+ 1 at. %, Na
+ 2 at. % single crystals were obtained by the Czochralski grown method. The structure of the crystals is tetragonal and belongs to the I4
1/a space group. The EPR angular dependencies allowed us to determine the SH parameters (in the crystallographic axis system) and to determine the symmetry of the substituted cerium ions. EPR measurements have shown that one isolated Ce
3+ center of high C
4 symmetry could be found in the BaWO
4 single crystal and two or more others of lower symmetry, C
2, could be identified, depending on crystal doping and co-doping. In addition, in BaWO
4: Ce single crystals co-doped with sodium ions, we have observed EPR lines originating from cerium ions at the C
2v symmetry site. The introduction of sodium ions causes the crystal lattice to be better ordered by increasing the number of cerium ions in C
4 symmetry. Photoluminescence spectrum from the Ce
3+ ions was not found. Only excitonic and defect centers luminescence in a range of 280 nm and 400–600 nm, respectively, have been detected. In a paper by Wlodarczyk et al. [
19], optical spectroscopy of the above crystals has been analyzed under different values of external pressure. The authors of reference 19 have concluded that the observed luminescence has an excitonic character, independent of applied pressure. This is related to the location of Ce
3+ 5d levels in the conduction band of the material. The luminescence is strongly temperature quenched, with a relatively small activation energy of a few meV [
19]. Although Ce
3+ does not show any emission centers, it contributes to the efficiency of exciton formation. It was observed in [
19] that in crystals doped with Ce
3+, the exciton emission intensity is higher than in pure crystals.