A Facile and Eco-Friendly Method for the Synthesis of Sulfonamide and Sulfonate Carboxylic Acid Derivatives—X-ray Structure, Hirshfeld Analysis and Spectroscopic Characterizations
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. X-ray Measurements
2.3. General Method for Synthesis of Sulfonamide Carboxylic Acid 4a-c and 5a-c
2.3.1. 2-(4-Methylphenylsulphonamido) Acetic Acid, 4a (Figure S1, Supplementary Materials)

2.3.2. 3-((4-Methylphenyl)sulfonamido)propanoic Acid, 4b (Figure S2, Supplementary Materials)

2.3.3. 4-((4-Methylphenyl)sulfonamido)butanoic Acid, 4c (Figure S3, Supplementary Materials)

2.3.4. 4-((4-methylphenylsulfonyl)oxy)benzoic Acid, 5a (Figure S4, Supplementary Materials)

2.3.5. 4-((4-Methylphenyl)sulfonamido)benzoic Acid, 5b (Figure S5, Supplementary Materials)

2.3.6. 2-(4-((4-Methylphenyl)sulfonamido)phenyl)acetic Acid, 5c (Figure S6, Supplementary Materials)

2.4. General Method for Preparation of 7a-b
2.4.1. 4-((2-Morpholinoethyl)carbamoyl)phenyl 4-methylbenzenesulfonate, 7a (Figure S7, Supplementary Materials)

2.4.2. 4-((4-Methylphenyl)sulfonamido)-N-(2-morpholinoethyl)benzamide, 7b (Figure S8, Supplementary Materials)

3. Results and Discussion
3.1. Chemistry

3.2. X-ray Structure Determination
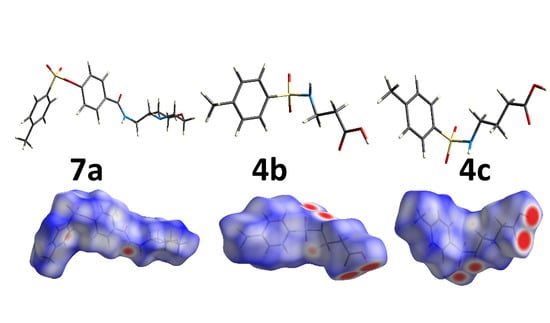
3.3. Hirshfeld Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Török, E.; Moran, E.; Cooke, F. Oxford Handbook of Infectious Diseases and Microbiology; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Selvam, P.; Chandramohan, M.; Clercq, E.D.; Witvrouw, M.; Pannecouque, C. Synthesis and anti-HIV activity of 4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)amino]-N(4,6-dimethyl-2-pyrimidinyl)-benzene sulfonamide and its derivatives. Eur. J. Pharm. Sci. 2001, 14, 313–316. [Google Scholar] [CrossRef]
- Stokes, S.S.; Albert, R.; Buurman, E.T.; Andrews, B.; Shapiro, A.B.; Green, O.M.; McKenzie, A.R.; Otterbein, L.R. Inhibitors of the acetyltransferase domain of N-acetylglucosamine-1-phosphate-uridylyltransferase/glucosamine-1-phosphate-acetyltransferase (GlmU). Part 2: Optimization of physical properties leading to antibacterial aryl sulfonamides. Bioorg. Med. Chem. Lett. 2012, 22, 7019–7023. [Google Scholar] [CrossRef] [PubMed]
- Abbate, F.; Casini, A.; Owa, T.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg. Med. Chem. Lett. 2004, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ezabadi, I.R.; Camoutsis, C.; Zoumpoulakis, P.; Geronikaki, A.; Soković, M.; Glamočilija, J.; Ćirić, A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg. Med. Chem. 2008, 16, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Zareef, M.; Iqbal, R.; Gamboa, N.; de Dominguez, A.; Rodrigues, J.; Zaizi, J.H.; Arfan, M.; Supuran, C. Synthesis and antimalarial activity of novel chiral and achiral benzenesulfonamides bearing 1, 3, 4-oxadiazole moieties. J. Enzym. Inhib. Med. Chem. 2007, 22, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranix, B.R.; Lavalle, J.F.; Sevigny, G.; Telle, J.; Perron, V.; Leberre, N.; Harbart, D.; Wu, J.J. Lysine sulfonamides as novel HIV-protease inhibitors: Nε-Acyl aromatic alpha-amino acids. Bioorg. Med. Chem. Lett. 2006, 16, 3459–3462. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Scozzafava, A.; Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 2003, 23, 146–189. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T.; Casini, A.; Scozzafava, A. Protease inhibitors of the sulfonamide type: Anticancer, antiinflammatory, and antiviral agents. Med. Res. Rev. 2003, 5, 535–558. [Google Scholar] [CrossRef]
- Chiaramonte, N.; Romanelli, M.N.; Teodori, E.; Supuran, C.T. Amino acids as building blocks for carbonic anhydrase inhibitors. Metabolites 2018, 8, 36. [Google Scholar] [CrossRef]
- Chinthakindi, P.K.; Arvidsson, P.I. Sulfonyl fluorides (SFs): More than click reagents. Eur. J. Org. Chem. 2018, 2018, 3648–3666. [Google Scholar] [CrossRef]
- Kim, J.-G.; Jang, D.O. Mild and efficient indium metal catalyzed synthesis of sulfonamides and sulfonic esters. Synlett 2007, 16, 2501–2504. [Google Scholar]
- Jafarpour, M.; Rezaeifard, A.; Golshani, T. Efficient organic transformations mediated by ZrOCl2·8H2O in Water. Phosphorus Sulfur Silicon Relat. Elem. 2010, 186, 140–148. [Google Scholar] [CrossRef]
- Luca, L.D.; Giacomelli, G. An Easy Microwave-assisted synthesis of sulfonamides directly from sulfonic acids. J. Org. Chem. 2008, 73, 3967–3969. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.N.S.; Khalafi-Nezhad, A.; Asrari, Z.; Behrouz, S.; Amini, Z.; Behrouz, M. One-pot synthesis of sulfonamides from primary and secondary amine derived sulfonate salts using cyanuric chloride. Synthesis 2009, 23, 3983–3988. [Google Scholar] [CrossRef]
- Veisi, H.; Ghorbani-Vagheic, R.; Hemmati, S.; Mahmoodi, J. Convenient One-Pot Synthesis of sulfonamides and sulfonyl azides from thiols using N-chlorosuccinimide. Synlett 2011, 16, 2315–2320. [Google Scholar] [CrossRef]
- Caddick, S.; Wilden, J.D.; Judd, D.B. Direct synthesis of sulfonamides and activated sulfonate esters from sulfonic acids. J. Am. Chem. Soc. 2004, 126, 1024–1025. [Google Scholar] [CrossRef]
- Flegeau, E.F.; Harrison, J.M.; Willis, M.C. One-Pot Sulfonamide synthesis exploiting the palladium-catalyzed sulfination of aryl iodides. Synlett 2016, 27, 101–105. [Google Scholar] [CrossRef]
- Woolven, H.; González-Rodríguez, C.; Marco, I.; Thompson, A.L.; Willis, M.C. DABCO-bis(sulfur dioxide), DABSO, as a convenient source of sulfur dioxide for organic synthesis: Utility in sulfonamide and sulfamide preparation. Org. Lett. 2011, 13, 4876–4878. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Abdel-Fattah, A.A.A.; Vakulenko, A.V.; Tao, H. N-Sulfonylbenzotriazoles as advantageous reagents for C-sulfonylation. J. Org. Chem. 2005, 70, 9191–9197. [Google Scholar] [CrossRef]
- Wright, S.W.; Hallstrom, K.N. A Convenient preparation of heteroaryl sulfonamides and sulfonyl fluorides from heteroaryl thiols. J. Org. Chem. 2006, 71, 1080–1084. [Google Scholar] [CrossRef]
- Pandya, R.; Murashima, T.; Tedeschi, L.; Barrett, A.G.M. Facile one-pot synthesis of aromatic and heteroaromatic sulfonamides. J. Org. Chem. 2003, 68, 8274–8276. [Google Scholar] [CrossRef]
- Andersen, K.K. Comprehensive Organic Chemistry; Jones, D.N., Ed.; Pergamon Press: Oxford, UK, 1979; Volume 3. [Google Scholar]
- Holmes, T.J.J.; Lawton, R.G. Preparation of non-symmetrical p-benzoquinone diimines for evaluation as protein cleavage reagents. J. Org. Chem. 1983, 48, 3146–3150. [Google Scholar] [CrossRef]
- Yasuhara, A.; Kameda, M.; Sakamoto, T. Selective Monodesulfonylation of N, N-disulfonylarylamines with tetrabutylammonium fluoride. Chem. Pharm. Bull. 1999, 47, 809–812. [Google Scholar] [CrossRef]
- Low, C.M.R.; Broughton, H.B.; Kalindjian, S.B.; McDonald, I.M. Novel oxathiazinones as gastrin ligands: Unexpected products from the Scho-Mén-Baumann reaction of arylsuphonyl chlorides with derivatives of aspartic acids. Bioorg. Med. Chem. Lett. 1992, 2, 325–330. [Google Scholar] [CrossRef]
- Hu, W.; Guo, Z.; Chu, F.; Bai, A.; Yi, X.; Cheng, G.; Li, J. Synthesis and biological evaluation of substituted 2-sulfonyl-phenyl-3-phenyl-indoles: A new series of selective COX-2 inhibitors. Bioorg. Med. Chem. 2003, 11, 1153–1160. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Fin, M.G.; Folkin, V.V.; Kolb, H.C.; Sharpless, K.B. “On water”: Unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, U.M. Stereoselective organic reactions in water. Chem. Rev. 2002, 102, 2751–2772. [Google Scholar] [CrossRef]
- Deng, X.; Mani, N.S. A facile, environmentally benign sulfonamide synthesis in water. Green Chem. 2006, 8, 835–838. [Google Scholar] [CrossRef]
- Kumar, A.; Jad, Y.E.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Green solid-phase peptide synthesis 4. δ-valerolactone and N-formylmorpholine as green solvents for solid-phase peptide synthesis. Tetrahedron Lett. 2017, 58, 2986–2988. [Google Scholar] [CrossRef]
- Jad, Y.E.; Govender, T.; Kruger, H.G.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Green solid-phase peptide synthesis (GSPPS) 3. Green solvents for Fmoc removal in peptide chemistry. Org. Process Res. Dev. 2017, 21, 365–369. [Google Scholar] [CrossRef]
- Jad, Y.E.; Acosta, G.; Govender, T.; Kruger, H.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Green solid-phase peptide synthesis-2.1, 2-Methytetrahydrofuran and ethyl acetate for solid-phase peptide synthesis under green conditions. ACS Sustain. Chem. Eng. 2016, 4, 6809–6814. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Jad, Y.E.; Kumar, A.; El-Faham, A.; Collins, J.M.; Basso, A.; de la Torre, B.G.; Albericio, F. Greening the solid-phase peptide synthesis process. 2-MeTHF for the incorporation of the first amino acid and precipitation of peptides after global deprotection. Org. Process Res. Dev. 2018, 22, 1809–1816. [Google Scholar]
- SAINT, version 4; Siemens Analytical X-ray Instruments Inc.: Madison, WI, USA, 1995.
- Sheldrick, G.M. SADABS; Program for Empirical Absorption Correction of Area Detector Data; University of Goettingen: Goettingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer; University of Western Australia: Perth, Australia, 2012; version 3.1. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Fidan, I.; Salmas, R.E.; Arslan, M.; Sentürk, M.; Durdagi, S.; Ekinci, D.; Sentürk, E.; Cosgun, S.; Supuran, C.T. Carbonic anhydrase inhibitors: Design, synthesis, kinetic, docking and molecular dynamics analysis of novel glycine and phenylalanine sulfonamide derivatives. Bioorg. Med. Chem. 2015, 23, 7353–7358. [Google Scholar] [CrossRef]
- Hill, R.R.; Moore, S.A.; Roberts, D.R. The Photochemistry of N-p-Toluenesulfonyl peptides: The peptide bond as an electron donor. Photochem. Photobiol. 2005, 81, 1439–1446. [Google Scholar] [CrossRef]
- Korsager, S.; Taaning, R.H.; Skrydstrup, T. Effective palladium-catalyzed hydroxycarbonylation of aryl halides with substoichiometric carbon monoxide. J. Am. Chem. Soc. 2013, 135, 2891–2894. [Google Scholar] [CrossRef]
- Mustafa, G.; Khan, I.U.; Ashraf, M.; Afzal, I.; Shahzad, S.A.; Shafiq, M. Synthesis of new sulfonamides as lipoxygenase inhibitors. Bioorg. Med. Chem. 2012, 20, 2535–2539. [Google Scholar] [CrossRef]
- Subiros-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. Eur. J. 2009, 15, 9394–9403. [Google Scholar] [CrossRef]









| 4b | 4c | 7a | |
|---|---|---|---|
| Empirical formula | C10H13NO4S | C11H15NO4S | C20H24N2O5S |
| Formula weight | 243.27 | 257.3 | 404.47 |
| Temperature/K | 293(2) | ||
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P-1 | P21/c | P21/c |
| a/Å | 5.2492(4) | 10.9634(7) | 17.4251(18) |
| b/Å | 10.7610(7) | 5.4574(3) | 5.2003(5) |
| c/Å | 11.6185(8) | 21.2783(14) | 22.117(2) |
| α/° | 115.002(2) | 90 | 90 |
| β/° | 101.701(2) | 100.633(3) | 99.179(5) |
| γ/° | 90.120(2) | 90 | 90 |
| Volume/Å3 | 579.57(7) | 1251.26(13) | 1978.5(3) |
| Z | 2 | 4 | 4 |
| ρcalc g/cm3 | 1.394 | 1.366 | 1.358 |
| μ/mm−1 | 0.278 | 0.261 | 0.198 |
| F(000) | 256 | 544 | 856 |
| Crystal size/mm3 | 0.51 × 0.28 × 0.25 | 0.56 × 0.42 × 0.05 | 0.03 × 0.09 × 0.65 |
| Radiation | Mo-Kα (λ = 0.71073 Å) | ||
| 2Θ range for data collection/° | 4.2 to 66.42 | 4.9 to 52 | 4.08 to 50.00 |
| Index ranges | −8 ≤ h ≤ 8 −16 ≤ k ≤ 16 −17 ≤ l ≤ 17 | −12 ≤ h ≤ 13 −6 ≤ k ≤ 6 −26 ≤ l ≤ 26 | −20 ≥ h ≤ 20 −6 ≤ k ≤ 6 −26 ≤ l ≤ 26 |
| Reflections collected | 33,710 | 13,336 | 39,573 |
| Independent reflections | 4428 [Rint = 0.0582] | 2455 [Rint = 0.0906] | 3479 [Rint = 0.2468] |
| Data/restraints/parameters | 4428/0/154 | 2455/0/163 | 3479/0/254 |
| Goodness-of-fit on F2 | 1.019 | 1.028 | 1.013 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0558, wR2 = 0.1281 | R1 = 0.0708, wR2 = 0.1726 | R1 = 0.0761, wR2 = 0.1492 |
| Final R indexes [all data] | R1 = 0.1017, wR2 = 0.1478 | R1 = 0.1027, wR2 = 0.1919 | R1 = 0.1787, wR2 = 0.1967 |
| Largest diff. peak/hole/e Å−3 | 0.40/−0.29 | 0.42/−0.36 | −0.31/0.40 |
| CCDC | 1524756 | 1524754 | 1524879 |
| S1–O1 | 1.4352(15) | S1–N1 | 1.6187(17) |
| O3–C10 | 1.309(3) | N1–C8 | 1.467(2) |
| C1–C6 | 1.387(3) | C3–C4 | 1.388(3) |
| C4–C5 | 1.376(3) | C8–C9 | 1.502(3) |
| S1–O2 | 1.4314(16) | S1–C6 | 1.7564(17) |
| O4–C10 | 1.207(3) | C1–C2 | 1.383(3) |
| C2–C3 | 1.382(3) | C3–C7 | 1.511(4) |
| C5–C6 | 1.388(3) | C9–C10 | 1.491(3) |
| O1–S1–O2 | 119.67(9) | C2–C3–C7 | 120.2(2) |
| O2–S1–N1 | 106.58(9) | C4–C5–C6 | 119.3(2) |
| S1–N1–C8 | 119.30(14) | C1–C6–C5 | 120.37(17) |
| C2–C3–C4 | 118.6(2) | O3–C10–O4 | 123.3(2) |
| C3–C4–C5 | 121.3(2) | O1–S1–C6 | 108.38(8) |
| S1–C6–C5 | 119.91(15) | N1–S1–C6 | 107.99(8) |
| C8–C9–C10 | 113.16(19) | C1–C2–C3 | 121.2(2) |
| O4–C10–C9 | 123.5(2) | C4–C3–C7 | 121.2(2) |
| O1–S1–N1 | 105.43(9) | S1–C6–C1 | 119.66(14) |
| O2–S1–C6 | 108.28(9) | N1–C8–C9 | 107.99(17) |
| C2–C1–C6 | 119.23(19) | O3–C10–C9 | 113.2(2) |
| D–H···A | D–H (Å) | H···A (Å) | D···A (Å) | D–H···A (°) |
|---|---|---|---|---|
| 4b | ||||
| N1–H1N1…O1 ii | 0.74(2) | 2.26(2) | 2.970(2) | 161(2) |
| O3–H1O3…O4 iii | 0.79(4) | 1.88(4) | 2.656(3) | 170(4) |
| 4c | ||||
| N1–H1N1…O1 iv | 0.82(5) | 2.17(4) | 2.963(4) | 166(5) |
| O3–H1O3…O4 v | 0.85(5) | 1.84(5) | 2.668(4) | 167(5) |
| 7a | ||||
| N1–H1B...O4 i | 0.86 | 2.20 | 3.025(6) | 159.7 |
| C9–H9A…O2 i | 0.93 | 2.75 | 3.511(7) | 139.4 |
| S1–O1 | 1.431(3) | S1–N1 | 1.618(3) |
| O3–C11 | 1.323(5) | N1–C8 | 1.472(5) |
| C1–C6 | 1.376(5) | C3–C4 | 1.377(6) |
| C4–C5 | 1.370(6) | C8–C9 | 1.497(5) |
| C10–C11 | 1.490(5) | S1–C6 | 1.758(4) |
| S1–O2 | 1.426(3) | C1–C2 | 1.381(6) |
| O4–C11 | 1.212(4) | C3–C7 | 1.506(6) |
| C2–C3 | 1.395(6) | C9–C10 | 1.511(6) |
| C5–C6 | 1.391(5) | ||
| O1–S1–O2 | 119.50(17) | C4–C5–C6 | 119.7(3) |
| O2–S1–N1 | 106.80(18) | C1–C6–C5 | 120.2(4) |
| S1–N1–C8 | 119.0(2) | C9–C10–C11 | 113.9(3) |
| C2–C3–C4 | 117.3(4) | O4–C11–C10 | 124.3(3) |
| C3–C4–C5 | 121.9(4) | O1–S1–C6 | 109.48(18) |
| S1–C6–C5 | 119.9(3) | N1–S1–C6 | 107.46(17) |
| C8–C9–C10 | 110.7(3) | C1–C2–C3 | 122.2(4) |
| O3–C11–C10 | 113.2(3) | C4–C3–C7 | 121.7(4) |
| O1–S1–N1 | 105.28(17) | S1–C6–C1 | 119.8(3) |
| O2–S1–C6 | 107.73(16) | N1–C8–C9 | 110.4(3) |
| C2–C1–C6 | 118.8(4) | O3–C11–O4 | 122.5(3) |
| C2–C3–C7 | 121.0(4) |
| S1–O1 | 1.428(4) | S1–O2 | 1.430(4) |
| O3–C8 | 1.422(6) | O4–C14 | 1.232(6) |
| N1–C14 | 1.333(6) | N1–C15 | 1.459(6) |
| N2–C20 | 1.443(8) | C1–C2 | 1.359(9) |
| C3–C4 | 1.399(9) | C3–C7 | 1.500(9) |
| C8–C9 | 1.372(8) | C8–C13 | 1.369(7) |
| C11–C12 | 1.386(6) | C11–C14 | 1.500(7) |
| C17–C18 | 1.495(9) | C19–C20 | 1.513(9) |
| C1–C6 | 1.392(9) | C2–C3 | 1.384(9) |
| C4–C5 | 1.379(9) | C5–C6 | 1.381(8) |
| C9–C10 | 1.381(8) | C10–C11 | 1.392(7) |
| C12–C13 | 1.365(7) | C15–C16 | 1.516(7) |
| S1–O3 | 1.595(4) | S1–C6 | 1.747(6) |
| O5-C18 | 1.416(8) | O5–C19 | 1.430(9) |
| N2–C16 | 1.454(6) | N2–C17 | 1.471(7) |
| O1-S1-O2 | 120.4(3) | O3–C8–C9 | 118.3(5) |
| O2–S1–O3 | 107.6(2) | C8–C9–C10 | 118.1(5) |
| S1–O3–C8 | 118.9(3) | C10–C11–C14 | 123.1(4) |
| C16–N2–C17 | 111.3(4) | C8–C13–C12 | 118.7(4) |
| C2–C1–C6 | 119.0(5) | N1–C14–C11 | 116.8(4) |
| C2–C3–C7 | 122.2(6) | N2–C17–C18 | 110.2(5) |
| C4–C5–C6 | 119.4(6) | N2–C20–C19 | 111.5(5) |
| C1–C6–C5 | 120.6(6) | O1–S1–C6 | 110.0(3) |
| C9–C8–C13 | 122.6(5) | O3–S1–C6 | 105.5(2) |
| C10–C11–C12 | 118.7(5) | C14–N1–C15 | 121.6(4) |
| C11–C12–C13 | 121.2(5) | C17–N2–C20 | 107.0(4) |
| O4–C14–C11 | 120.7(4) | C2–C3-C4 | 117.9(6) |
| N2–C16–C15 | 110.5(4) | C3-C4–C5 | 120.8(6) |
| O5–C19–C20 | 110.8(5) | S1–C6–C5 | 118.8(5) |
| O1–S1–O3 | 103.4(2) | O3–C8–C13 | 119.1(4) |
| O2–S1–C6 | 108.9(3) | C9–C10–C11 | 120.7(5) |
| C18–O5–C19 | 110.0(5) | C12–C11–C14 | 118.2(4) |
| C16–N2–C20 | 111.3(4) | O4–C14–N1 | 122.5(5) |
| C1–C2–C3 | 122.3(6) | N1–C15–C16 | 113.1(4) |
| C4–C3–C7 | 119.9(5) | O5–C18–C17 | 111.6(5) |
| S1–C6–C1 | 120.6(4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almarhoon, Z.; Soliman, S.M.; Ghabbour, H.A.; El-Faham, A. A Facile and Eco-Friendly Method for the Synthesis of Sulfonamide and Sulfonate Carboxylic Acid Derivatives—X-ray Structure, Hirshfeld Analysis and Spectroscopic Characterizations. Crystals 2019, 9, 35. https://doi.org/10.3390/cryst9010035
Almarhoon Z, Soliman SM, Ghabbour HA, El-Faham A. A Facile and Eco-Friendly Method for the Synthesis of Sulfonamide and Sulfonate Carboxylic Acid Derivatives—X-ray Structure, Hirshfeld Analysis and Spectroscopic Characterizations. Crystals. 2019; 9(1):35. https://doi.org/10.3390/cryst9010035
Chicago/Turabian StyleAlmarhoon, Zainab, Saied M. Soliman, Hazem A. Ghabbour, and Ayman El-Faham. 2019. "A Facile and Eco-Friendly Method for the Synthesis of Sulfonamide and Sulfonate Carboxylic Acid Derivatives—X-ray Structure, Hirshfeld Analysis and Spectroscopic Characterizations" Crystals 9, no. 1: 35. https://doi.org/10.3390/cryst9010035
APA StyleAlmarhoon, Z., Soliman, S. M., Ghabbour, H. A., & El-Faham, A. (2019). A Facile and Eco-Friendly Method for the Synthesis of Sulfonamide and Sulfonate Carboxylic Acid Derivatives—X-ray Structure, Hirshfeld Analysis and Spectroscopic Characterizations. Crystals, 9(1), 35. https://doi.org/10.3390/cryst9010035