Characterization of Coals and Coal Ashes with High Si Content Using Combined Second-Derivative Infrared Spectroscopy and Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal Samples
2.2. Ash Samples Preparation
2.3. Characterization of Organic and Inorganic Components in Coals and Coal Ashes
3. Results and Discussion
3.1. FTIR of Coals and Coal Ashes
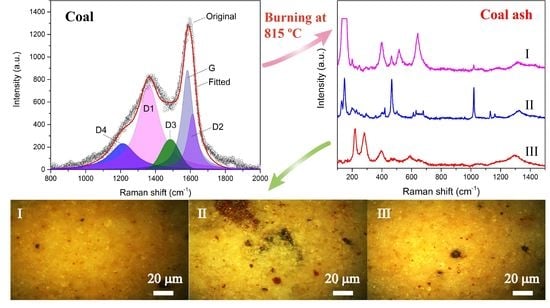
3.2. Raman Spectra of Coals and Coal Ashes
3.3. XRD of Coals and Coal Ashes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, R. Advanced coal characterization: A review. Energy Fuels 2007, 21, 451–460. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Methods for characterization of composition of fly ashes from coal-fired power stations: A critical overview. Energy Fuels 2005, 19, 1084–1098. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Wang, Z.; Li, Q.; Whiddon, R.; He, Y.; Cen, K. Pyrolysis behavior of a typical Chinese sub-bituminous Zhundong coal from moderate to high temperatures. Fuel 2016, 185, 701–708. [Google Scholar] [CrossRef]
- Huan, X.; Tang, Y.G.; Xu, J.J.; Lan, C.Y.; Wang, S.Q. Structural characterization of graphenic material prepared from anthracites of different characteristics: A comparative analysis. Fuel Process. Technol. 2019, 183, 8–18. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Qian, K.; Yang, Z.; Yang, H.; Li, Y.; Chen, H. Evolution of char structure during mengdong coal pyrolysis: Influence of temperature and K2CO3. Fuel Process. Technol. 2017, 159, 178–186. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via Micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Qiu, P.H.; Xie, X.; Chen, X.Y.; Lin, D.; Sun, S.Z. Impacts of chemical fractionation on Zhundong coal’s chemical structure and pyrolysis reactivity. Fuel Process. Technol. 2017, 155, 144–152. [Google Scholar] [CrossRef]
- Martínez-Tarazona, M.R.; Spears, D.A.; Palacios, J.; Martínez-Alonso, A.; Tascón, J.M.D. Mineral matter in coals of different rank from the Asturian Central basin. Fuel 1992, 71, 367–372. [Google Scholar] [CrossRef]
- Baruah, M.K.; Kotoky, P.; Borah, G.C. Distribution and nature of organic/mineral bound elements in Assam coals, India. Fuel 2003, 82, 1783–1791. [Google Scholar] [CrossRef]
- Ibarra, J.; Palacios, J.; de Andrés, A.M. Analysis of coal and char ashes and their ability for sulphur retention. Fuel 1989, 68, 861–867. [Google Scholar] [CrossRef]
- Painter, P.C.; Coleman, M.M.; Jenkins, R.G.; Whang, P.W.; Walker, P.L. Fourier Transform Infrared study of mineral matter in coal. A novel method for quantitative mineralogical analysis. Fuel 1978, 57, 337–344. [Google Scholar] [CrossRef]
- Mukherjee, S.; Srivastava, S. Minerals transformations in northeastern region coals of India on heat treatment. Energy Fuels 2006, 20, 1089–1096. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Dyczek, J.; Deja, J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta Part A 2014, 132, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, J.; Munoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Koch, A.; Krzton, A.; Finqueneisel, G.; Heintz, O.; Weber, J.V.; Zimny, T. A study of carbonaceous char oxidation in air by semi-quantitative FTIR spectroscopy. Fuel 1998, 77, 563–569. [Google Scholar] [CrossRef]
- Sheng, C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H. Volatile-char interactions: Roles of in situ volatiles with distinctly-different chemistry in determining char structure and reactivity. Proc. Combust. Inst. 2019, 37, 2749–2755. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.I.; Li, C.Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Wu, H.; Yip, K.; Tian, F.; Xie, Z.; Li, C.Z. Evolution of char structure during the steam gasification of biochars produced from the pyrolysis of various mallee biomass components. Ind. Eng. Chem. Res. 2009, 48, 10431–10438. [Google Scholar] [CrossRef]
- Xu, J.; Tang, H.; Su, S.; Liu, J.; Xu, K.; Qian, K.; Wang, Y.; Zhou, Y.; Hu, S.; Zhang, A.; et al. A study of the relationships between coal structures and combustion characteristics: The insights from micro-Raman spectroscopy based on 32 kinds of Chinese coals. Appl. Energy 2018, 212, 46–56. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Edwards, H.G.; Moens, L. A decade of Raman spectroscopy in art and archaeology. Chem. Rev. 2007, 107, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Sampaio, C.; Guedes, A.; de Vallejuelo, S.F.O.; Madariaga, J. Multianalytical approaches to the characterisation of minerals associated with coals and the diagnosis of their potential risk by using combined instrumental microspectroscopic techniques and thermodynamic speciation. Fuel 2012, 94, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Valentim, B.; Shreya, N.; Paul, B.; Gomes, C.S.; Sant’Ovaia, H.; Guedes, A.; Ribeiro, J.; Flores, D.; Pinho, S.; Suárez-Ruiz, I. Characteristics of ferrospheres in fly ashes derived from Bokaro and Jharia (Jharkand, India) coals. Int. J. Coal Geol. 2016, 153, 52–74. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Sanz, A.; Flores, D.; Noronha, F. Characterization of fly ash from a power plant and surroundings by micro-Raman spectroscopy. Int. J. Coal Geol. 2008, 73, 359–370. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Ibarra, J.; Moliner, R.; Bonet, A.J. FT-i.r. investigation on char formation during the early stages of coal pyrolysis. Fuel 1994, 73, 918–924. [Google Scholar] [CrossRef]
- Kumar, R.; Bansal, V.; Badhe, R.; Madhira, I.S.S.; Sugumaran, V.; Ahmed, S.; Christopher, J.; Patel, M.B.; Basu, B. Characterization of Indian origin oil shale using advanced analytical techniques. Fuel 2013, 113, 610–616. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Saikia, N.; Bharali, D.; Sengupta, P.; Bordoloi, D.; Goswamee, R.; Saikia, P.; Borthakur, P. Characterization, beneficiation and utilization of a kaolinite clay from Assam, India. Appl. Clay Sci. 2003, 24, 93–103. [Google Scholar] [CrossRef]
- Shoval, S.; Beck, P. Thermo-FTIR spectroscopy analysis as a method of characterizing ancient ceramic technology. J. Therm. Anal. Calorim. 2005, 82, 609–616. [Google Scholar] [CrossRef]
- Fabbri, B.; Gualtieri, S.; Leonardi, C. Modifications induced by the thermal treatment of kaolin and determination of reactivity of metakaolin. Appl. Clay Sci. 2013, 73, 2–10. [Google Scholar] [CrossRef]
- Yin, Y.; Yin, J.; Zhang, W.; Tian, H.; Hu, Z.; Ruan, M.; Xu, H.; Liu, L.; Yan, X.; Chen, D. FT-IR and micro-Raman spectroscopic characterization of minerals in high-calcium coal ashes. J. Energy Inst. 2018, 91, 389–396. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal treatment of kaolin: The effect of mineralogy on the pozzolanic activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Ramasamy, V.; Rajkumar, P.; Ponnusamy, V. Depth wise analysis of recently excavated Vellar river sediments through FTIR and XRD studies. Indian J. Phys. 2009, 83, 1295–1308. [Google Scholar] [CrossRef]
- De Benedetto, G.; Laviano, R.; Sabbatini, L.; Zambonin, P. Infrared spectroscopy in the mineralogical characterization of ancient pottery. J. Cult. Herit. 2002, 3, 177–186. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G.; Dimitrovska, S. Minerals from Macedonia: XIV. Identification of some sulfate minerals by vibrational (infrared and Raman) spectroscopy. Vib. Spectrosc. 2005, 39, 229–239. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G.; Kaitner, B. Minerals from Macedonia. XXIV. Spectra-Structure characterization of tectosilicates. J. Mol. Struct. 2009, 924, 413–419. [Google Scholar] [CrossRef]
- Pusz, S.; Krztoń, A.; Komraus, J.; Martinez-Tarazona, M.; Martinez-Alonso, A.; Tascon, J. Interactions between organic matter and minerals in two bituminous coals of different rank. Int. J. Coal Geol. 1997, 33, 369–386. [Google Scholar] [CrossRef]
- Shoval, S.; Panczer, G.; Boudeulle, M. Study of the occurrence of titanium in kaolinites by micro-Raman spectroscopy. Opt. Mater. 2008, 30, 1699–1705. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Antunes, V.; Candeias, A.; Oliveira, M.J.; Longelin, S.; Serrão, V.; Seruya, A.I.; Coroado, J.; Dias, L.; Mirão, J.; Carvalho, M.L. Characterization of gypsum and anhydrite ground layers in 15th and 16th centuries Portuguese paintings by Raman spectroscopy and other techniques. J. Raman Spectrosc. 2014, 45, 1026–1033. [Google Scholar] [CrossRef]
- Ayora-Cañada, M.; Domínguez-Arranz, A.; Dominguez-Vidal, A. Raman Microspectroscopic study of Iberian pottery from the La Vispesa archaeological site, Spain. J. Raman Spectrosc. 2012, 43, 317–322. [Google Scholar] [CrossRef]
- De Faria, D.; Venâncio Silva, S.; De Oliveira, M. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Vassileva, C.G.; Vassilev, S.V. Behaviour of inorganic matter during heating of Bulgarian coals: 1. Lignites. Fuel Process. Technol. 2005, 86, 1297–1333. [Google Scholar] [CrossRef]
- Gridi-Bennadji, F.; Beneu, B.; Laval, J.-P.; Blanchart, P. Structural transformations of muscovite at high temperature by X-ray and neutron diffraction. Appl. Clay Sci. 2008, 38, 259–267. [Google Scholar] [CrossRef]
- Bryers, R.W. Fireside slagging, fouling, and high-temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels. Prog. Energy Combust. Sci. 1996, 22, 29–120. [Google Scholar] [CrossRef]






| Sample | Proximate Analysis (wt %, Air-Dried Basis) | Ultimate Analysis (wt %, Dry Basis) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | N | O a | S | |
| coal A | 11.12 | 25.84 | 27.95 | 35.09 | 40.15 | 3.43 | 0.71 | 26.09 | 0.55 |
| coal B | 16.55 | 17.88 | 29.43 | 36.14 | 43.06 | 4.02 | 0.83 | 30.24 | 0.42 |
| Sample | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | Na2O | MgO | SO3 | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| coal ash A | 48.11 | 21.58 | 7.35 | 10.13 | 1.66 | 2.30 | 2.34 | 2.31 | 0.36 | 1.18 |
| coal ash B | 40.45 | 15.01 | 8.7 | 11.99 | 0.99 | 3.85 | 3.56 | 9.08 | 0.77 | 1.06 |
| Organic or Mineral Matter | Absorption Band Frequencies/cm−1 |
|---|---|
| Kaolinite | 3696, 3619, 1101, 1032, 1009, 938, 913, 756, 696, 538, 470, 431 |
| Aliphatic –CH2 | 2921, 2851 |
| Aromatic C=C | 1606 |
| Quartz | 1165, 1089, 799, 779, 696, 509 a, 465 |
| Gypsum | 1146 a, 1114 a, 604 |
| Anhydrite | 1151 a, 1118, 679, 614, 595 |
| Calcite | 875 a |
| Feldspars | 645 a, 424 a |
| Amorphous silica | 1196 a |
| Muscovite | 1062 a, 482 a |
| Saponite | 660 |
| Metakaolinite | 560 |
| Nitrate | 1384 |
| Sample | Position (cm−1) | Ratio of Peak Areas | ||||||
|---|---|---|---|---|---|---|---|---|
| D4 | D1 | D3 | G | D2 | ID1/IG | ID1/IAll | IG/IAll | |
| Coal A | 1227.7 | 1366.3 | 1474.7 | 1583.4 | 1611.9 | 1.76 | 0.44 | 0.25 |
| Standard deviation | 14.4 | 4.4 | 8.5 | 0.7 | 0.8 | 0.09 | 0.03 | 0.01 |
| Coal B | 1234.9 | 1372.2 | 1481.4 | 1580.6 | 1610.4 | 1.95 | 0.48 | 0.25 |
| Standard deviation | 6.1 | 1.5 | 14.9 | 4.4 | 3.1 | 0.34 | 0.05 | 0.02 |
| Mineral | Characteristic Raman Bands/cm−1 |
|---|---|
| Anatase | 148, 199, 400, 517, 642 |
| Anhydrite | 420, 502, 612, 631, 678, 1021, 1132, 1164 |
| Quartz | 130, 205, 358, 467 |
| Hematite | 222, 281, 395, 591, 1295 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Yin, H.; Wu, Z.; Qi, C.; Tian, H.; Zhang, W.; Hu, Z.; Feng, L. Characterization of Coals and Coal Ashes with High Si Content Using Combined Second-Derivative Infrared Spectroscopy and Raman Spectroscopy. Crystals 2019, 9, 513. https://doi.org/10.3390/cryst9100513
Yin Y, Yin H, Wu Z, Qi C, Tian H, Zhang W, Hu Z, Feng L. Characterization of Coals and Coal Ashes with High Si Content Using Combined Second-Derivative Infrared Spectroscopy and Raman Spectroscopy. Crystals. 2019; 9(10):513. https://doi.org/10.3390/cryst9100513
Chicago/Turabian StyleYin, Yanshan, Huixia Yin, Zihua Wu, Caiwen Qi, Hong Tian, Wei Zhang, Zhangmao Hu, and Leihua Feng. 2019. "Characterization of Coals and Coal Ashes with High Si Content Using Combined Second-Derivative Infrared Spectroscopy and Raman Spectroscopy" Crystals 9, no. 10: 513. https://doi.org/10.3390/cryst9100513
APA StyleYin, Y., Yin, H., Wu, Z., Qi, C., Tian, H., Zhang, W., Hu, Z., & Feng, L. (2019). Characterization of Coals and Coal Ashes with High Si Content Using Combined Second-Derivative Infrared Spectroscopy and Raman Spectroscopy. Crystals, 9(10), 513. https://doi.org/10.3390/cryst9100513