Spin Transition in the Cu(hfac)2 Complex with (4-Ethylpyridin-3-yl)-Substituted Nitronyl Nitroxide Caused by the “Asymmetric” Structural Rearrangement of Exchange Clusters in the Heterospin Molecule
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Synthesis of Compounds
2.3. Magnetic Measurements
2.4. Crystal Structure Determinations
2.5. IR-Spectrum Experiments
2.6. Density Functional Theory (DFT) Calculations
3. Results
3.1. Synthesis of LEt
3.2. Magnetic and X-Ray Investigations for LEt
3.3. Magnetic and X-Ray Investigations for [Cu(hfac)2LEt]2
3.4. X-Ray Investigations for[[Cu(hfac)2]4(LEt)2]
3.5. IR Spectroscopy of [[Cu(hfac)2]4(LEt)2]
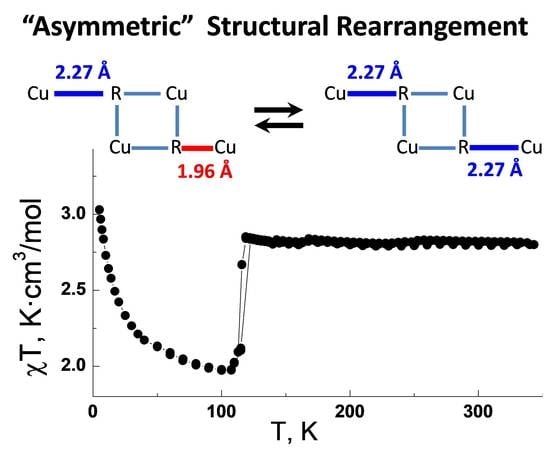
3.6. Magnetic Investigations for [[Cu(hfac)2]4(LEt)2]
3.7. Quantum-Chemical Calculations for [[Cu(hfac)2]4(LEt)2]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ovcharenko, V.I.; Bagryanskaya, E.G. Spin-Crossover Materials: Properties and Applications; Halcrow, M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar]
- Tretyakov, E.V.; Tolstikov, S.E.; Suvorova, A.O.; Polushkin, A.V.; Romanenko, G.V.; Bogomyakov, A.S.; Veber, S.L.; Fedin, M.V.; Stass, D.V. Crucial role of paramagnetic ligands for magnetostructural anomalies in “breathing crystals”. Inorg. Chem. 2012, 51, 9385–9394. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.I.; Romanenko, G.V.; Maryunina, K.Y.; Bogomyakov, A.S.; Gorelik, E.V. Thermally Induced Magnetic Anomalies in Solvates of the Bis(hexafluoroacetylacetonate) copper (II) Complex with Pyrazolyl-Substituted NitronylNitroxide. Inorg. Chem. 2008, 47, 9537–9552. [Google Scholar] [CrossRef]
- Lanfranc de Panthou, F.; Belorizky, E.; Calemczuk, R.; Luneau, D.; Marcenat, C.; Ressouche, E.; Turek, P.; Rey, P. A new type of thermally-induced spin transition associated with an equatorial. dblarw. axial conversion in a copper(II)-nitroxide cluster. J. Am. Chem. Soc. 1995, 117, 11247–11253. [Google Scholar]
- Lanfranc de Panthou, F.; Luneau, D.; Musin, R.N.; Öhrström, L.; Grand, A.; Turek, P.; Rey, P. Spin-Transition and Ferromagnetic Interactions in Copper(II) Complexes of a 3-Pyridyl-Substituted IminoNitroxide. Dependence of the Magnetic Properties upon Crystal Packing. Inorg. Chem. 1996, 35, 3484–3491. [Google Scholar] [CrossRef]
- Rey, P.; Ovcharenko, V.I. Copper(II) Nitroxide Molecular Spin-transition Complexes. In Magnetism: Molecules to Materials: 5 Volumes Set; Miller, J.S., Drillon, M., Eds.; Wiley-VCH: New York, NY, USA, 2003; pp. 41–63. [Google Scholar]
- Ovcharenko, V.I. Metal-nitroxide complexes: synthesis and Magnetostructural Correlations. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Hicks, R., Ed.; Wiley-VCH: New York, NY, USA, 2010; pp. 461–507. [Google Scholar]
- Halcrow, M. Spin-Crossover Materials: Properties and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar]
- Tolstikov, S.E.; Artiukhova, N.A.; Romanenko, G.V.; Bogomyakov, A.S.; Zueva, E.M.; Barskaya, I.Y.; Fedin, M.V.; Maryunina, K.Y.; Tretyakov, E.V.; Sagdeev, R.Z. Heterospin complex showing spin transition at room temperature. Polyhedron 2015, 100, 132–138. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Gao, Y.-Y.; Yang, M.-F.; Gao, T.; Ma, Y.; Wang, Q.-L.; Liao, D.-Z. Four new Cu-coordination compounds based on different nitronylnitroxide radicals: Structural design and magneto-structural correlations. Polyhedron 2013, 61, 105–111. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Guo, J.; Sun, G.; Li, L. Cu–Ln compounds based on nitronylnitroxide radicals: Synthesis, structure, and magnetic and fluorescence properties. Cryst. Eng. Comm. 2016, 19, 9345–9356. [Google Scholar] [CrossRef]
- Souza, D.A.; Florencio, A.S.; Soriano, S.; Calvo, R.; Sartoris, R.P.; Carneiro, J.W.d.M.; Sangregorio, C.; Novak, M.A.; Vaz, M.G.F. New copper(II)-radical one dimensional chain: Synthesis, crystal structure, EPR, magnetic properties and DFT calculations. Dalton Trans. 2009, 34, 6816–6824. [Google Scholar] [CrossRef]
- Souza, D.A.; Moreno, Y.; Ponzio, E.A.; Resende, J.A.; Jordao, A.K.; Cunha, A.C.; Ferreira, V.F.; Novak, M.A.; Vaz, M.G.F. Synthesis, crystal structure, magnetism and electrochemical properties of two copper(II) furoyltrifluoroacetonate complexes with nitroxide radical. Inorg. Chim. Acta 2011, 370, 469–473. [Google Scholar] [CrossRef]
- Caneschi, A.; Ferraro, F.; Gatteschi, D.; Rey, P.; Sessoli, R. Structure and magnetic properties of a chain compound formed by copper (II) and a tridentate nitronylnitroxide radical. Inorg. Chem. 1991, 30, 3162–3166. [Google Scholar] [CrossRef]
- Yeltsov, I.; Ovcharenko, V.; Ikorskii, V.; Romanenko, G.; Vasilevsky, S. Copper(II) Thenoyltrifluoroacetonate as Acceptor Matrix in Design of Heterospin Complexes. Polyhedron 2001, 20, 1215–1222. [Google Scholar] [CrossRef]
- Hirel, C.; Li, L.; Brough, P.; Vostrikova, K.; Pecaut, J.; Mehdaoui, B.; Bernard, M.; Turek, P.; Rey, P. New spin-transition-like-nitroxide species. Inorg. Chem. 2007, 46, 7545–7552. [Google Scholar] [CrossRef]
- Wang, K.-M.; Du, L.; Fang, R.-B.; Zhao, Q.-H. Synthesis and Crystal Structure of Copper(II)-Hexafluoro-Acetylacetonate Complexes with Pyridyl-Substituted Nitronyl and Imino-Nitroxide Radicals. J. Chem. Cryst. 2010, 40, 472–475. [Google Scholar] [CrossRef]
- Artiukhova, N.A.; Maryunina, K.Y.; Fokin, S.V.; Tretyakov, E.V.; Romanenko, G.V.; Polushkin, A.V.; Bogomyakov, A.S.; Sagdeev, R.Z.; Ovcharenko, V.I. Spirocyclic Derivatives of NitronylNitroxides in the Design Of Heterospin Cu(II) Complexes Manifesting Spin Transitions. Russ. Chem. Bull. 2013, 62, 2132–2140. [Google Scholar] [CrossRef]
- Wang, X.-F.; Licun Li, P.H.; Sutter, J.-P. [(Cu-Radical)2-Ln]: Structure and Magnetic Properties of a Hetero-tri-spin Chain of Rings (Ln = YIII, GdIII, TbIII, DyIII). Inorg. Chem. 2015, 54, 9664–9669. [Google Scholar] [CrossRef]
- Wang, X.-F.; Hu, P.; Li, Y.-G.; Li, L.-C. Construction of NitronylNitroxide-Based 3d–4f Clusters: Structure and Magnetism. Chem. Asian J. 2015, 10, 325–328. [Google Scholar] [CrossRef]
- Artiukhova, N.A.; Romanenko, G.V.; Letyagin, G.A.; Bogomyakov, A.S.; Tolstikov, S.E.; Ovcharenko, V.I. Spin transition characteristics of molecular solvates of CuII complexes with nitroxides: Sensitivity to the packing type. Russ. Chem. Bull 2019, 68, 732–742. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Kaplan, R.I. A Study of Bis(hexafluoroacetylacetonato)copper(II). Inorg. Chem. 1966, 5, 489–491. [Google Scholar] [CrossRef]
- Gueritte, F.; Guillou, C.; Husson, H.-P.; Kozielski, F.; Labriere, C.; Skoufias, D.; Tcherniuk, S.; Thal, C. Use of Derivatives of Indoles for the Treatment of Cancer. EP2266562A1, 29 December 2010. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502–395520. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Streltsov, S.V.; Petrova, M.V.; Morozov, V.A.; Romanenko, G.V.; Anisimov, V.I.; Lukzen, N.N. Interplay between lattice, orbital, and magnetic degrees of freedom in the chain-polymer Cu(II) breathing crystals. Phys. Rev. B 2013, 87, 024425–024425/6. [Google Scholar] [CrossRef]
- Morozov, V.A.; Petrova, M.V.; Lukzen, N.N. Exchange coupling transformations in Cu (II) heterospin complexes of “breathing crystals” under structural phase transitions. AIP Adv. 2015, 5, 087161. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Veber, S.L.; Suturina, E.A.; Fedin, M.V.; Boldyrev, K.N.; Maryunina, K.Y.; Sagdeev, R.Z.; Ovcharenko, V.I.; Gritsan, N.P.; Bagryanskaya, E.G. FTIR study of thermally induced magnetostructural transitions in breathing crystals. Inorg Chem. 2015, 54, 3446–3455. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Fukui, H.; Fueno, T. Molecular Orbital (MO) Theory for Magnetically Interacting Organic Compounds. Ab-Initio MO Calculations of the Effective Exchange Integrals For Cyclophane-Type Carbene Dimers. Chem. Lett. 1986, 15, 625–628. [Google Scholar] [CrossRef]
- Cho, D.; Ko, K.C.; Ikabata, Y.; Wakayama, K.; Yoshikawa, T.; Nakai, H.; Lee, J.Y. Effect of Hartree-Fock exact exchange on intramolecular magnetic coupling constants of organic diradicals. J. Chem. Phys. 2015, 142, 024318. [Google Scholar] [CrossRef]








| T (K) | 295 | 240 | 200 | 50 | |
|---|---|---|---|---|---|
| Cu−ONO | 2.193(2) | 2.128(2) | 2.062(5) | 1.963(9) | 1.979(8) |
| ∠CuORN | 127.8(2) | 126.53(2) | 126.2(4) | 122.0(7) | 124.9(7) |
| Cu−Ohfac | 1.965(2), 2.074(2) 2.105(2), 2.204(4) | 1.951(3), 2.123(3) 2.122(4), 2.152(3) | 1.921(5), 2.056(8) 2.182(7), 2.217(6) | 1.944(9), 1.987(9) 2.302(10), 2.338(10) | 1.961(9), 2.035(9) 2.270(10), 2.279(10) |
| Cu−N | 1.996(2) | 1.989(3) | 1.991(6) | 1.940(11) | 1.977(10) |
| ∠CN2O2−Py | 54.7 | 53.8 | 53.0 | 50.1 | 53.6 |
| ∠Py−Et | 6.7(5) | 9.8(5) | 7(2) | 16(1) | 12(2) |
| T, K | 295 | 240 | 200 | 154 | 150 | 100 | 50 | 240 * |
|---|---|---|---|---|---|---|---|---|
| Cu−OR (inn.) | 2.553(4) | 2.498(2) | 2.497(2) | 2.480(3) | 2.526(2), 2.413(2), 2.490(2) | 2.428(3), 2.424(3) | 2.406(3), 2.425(3) | 2.511(3) |
| Cu−OR (term.) | 2.276(4) | 2.287(2) | 2.287(2) | 2.285(4) | 2.308(2), 2.290(2), 2.290(2) | 1.954(3), 2.281(3) | 1.961(3), 2.270(3) | 2.276(3) |
| τ | 0.060 | 0.045 | 0.04650 | 0.042 | 0.228, 0.266, 0.239 | 0.375, 0.259 | 0.346, 0.253 | 0.263 |
| Cu−N | 1.998(4) | 2.016(2) | 2.016(2) | 2.009(4) | 2.008(3), 2.018(3), 2.016(3) | 2.015(4), 2.017(4) | 2.022(4), 2.021(4) | 2.005(3) |
| ∠CN2O2−Py | 56.3 | 56.7 | 56.7 | 57.0 | 53.5, 54.7, 60.3 | 54.9, 57.0 | 54.4, 56.6 | 56.2 |
| ∠Py−Et | 16.2 | 24.3 | 24.3 | 36.5 | 53.2, 41.6, 56.3 | 60.7, 63.6 | 61.1, 63.4 | 19.4 |
| T, K | J21 | J1 | J22 | J4 |
|---|---|---|---|---|
| 50 | 37.7 | 15.2 | 49.9 | −719.0 |
| 100 | 37.7 | 14.9 | 49.0 | −720.0 |
| 153 | 37.1 | 12.9 | 37.1 | 12.9 |
| 295 | 32.7 | 9.6 | 32.7 | 9.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artiukhova, N.; Romanenko, G.; Letyagin, G.; Bogomyakov, A.; Veber, S.; Minakova, O.; Petrova, M.; Morozov, V.; Ovcharenko, V. Spin Transition in the Cu(hfac)2 Complex with (4-Ethylpyridin-3-yl)-Substituted Nitronyl Nitroxide Caused by the “Asymmetric” Structural Rearrangement of Exchange Clusters in the Heterospin Molecule. Crystals 2019, 9, 285. https://doi.org/10.3390/cryst9060285
Artiukhova N, Romanenko G, Letyagin G, Bogomyakov A, Veber S, Minakova O, Petrova M, Morozov V, Ovcharenko V. Spin Transition in the Cu(hfac)2 Complex with (4-Ethylpyridin-3-yl)-Substituted Nitronyl Nitroxide Caused by the “Asymmetric” Structural Rearrangement of Exchange Clusters in the Heterospin Molecule. Crystals. 2019; 9(6):285. https://doi.org/10.3390/cryst9060285
Chicago/Turabian StyleArtiukhova, Natalia, Galina Romanenko, Gleb Letyagin, Artem Bogomyakov, Sergey Veber, Olga Minakova, Marina Petrova, Vitaliy Morozov, and Victor Ovcharenko. 2019. "Spin Transition in the Cu(hfac)2 Complex with (4-Ethylpyridin-3-yl)-Substituted Nitronyl Nitroxide Caused by the “Asymmetric” Structural Rearrangement of Exchange Clusters in the Heterospin Molecule" Crystals 9, no. 6: 285. https://doi.org/10.3390/cryst9060285
APA StyleArtiukhova, N., Romanenko, G., Letyagin, G., Bogomyakov, A., Veber, S., Minakova, O., Petrova, M., Morozov, V., & Ovcharenko, V. (2019). Spin Transition in the Cu(hfac)2 Complex with (4-Ethylpyridin-3-yl)-Substituted Nitronyl Nitroxide Caused by the “Asymmetric” Structural Rearrangement of Exchange Clusters in the Heterospin Molecule. Crystals, 9(6), 285. https://doi.org/10.3390/cryst9060285