Manufacturing and Characterization of Toughened Poly(lactic acid) (PLA) Formulations by Ternary Blends with Biopolyesters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing of Ternary PLA/PHB/PCL Blends
2.3. Mechanical Characterization
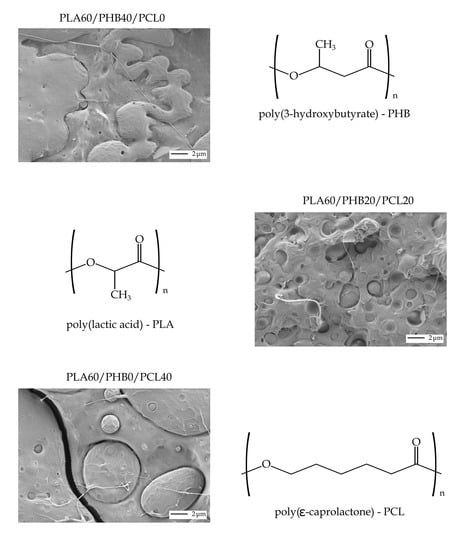
2.4. Morphology Characterization
2.5. Thermal and Thermomechanical Characterization
3. Results and Discussion
3.1. Mechanical Properties and Morphology of PLA/PHB/PCL Ternary Blends
3.2. Thermal Transitions and Thermo-Mechanical Behaviour of PLA/PHB/PCL Ternary Blends
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Nurul Fazita, M.; Jayaraman, K.; Bhattacharyya, D.; Mohamad Haafiz, M.; Saurabh, C.K.; Hussin, M.H.; HPS, A.K. Green composites made of bamboo fabric and poly(lactic) acid for packaging applications—A review. Materials 2016, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Fombuena, V.; Valles-Lluch, A.; Ellingham, T. Improvement of mechanical and biological properties of polycaprolactone loaded with hydroxyapatite and halloysite nanotubes. Mat. Sci. Eng. C Mater. 2017, 75, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Al-Duhaidahwi, H.R.H.; Al-Mulla, E.A.J.; Ali, H.A.A. Enhancement of properties and biodegradability of polybutylene succinate by epoxidized palm oil. Epitoanyag J. Silic. Based Compos. Mater. 2016, 68, 2–5. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Gilmer, C.; Wentzel, M.T.; Martello, M.T.; Kubo, T.; Wissinger, J.E. Sustainable polymers in the organic chemistry laboratory: Synthesis and characterization of a renewable polymer from δ-decalactone and l-lactide. J. Chem. Educ. 2013, 91, 131–135. [Google Scholar] [CrossRef]
- Mohanty, A.; Misra, M.; Drzal, L. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Torres-Tello, E.V.; Robledo-Ortiz, J.R.; Gonzalez-Garcia, Y.; Perez-Fonseca, A.A.; Jasso-Gastinel, C.F.; Mendizabal, E. Effect of agave fiber content in the thermal and mechanical properties of green composites based on polyhydroxybutyrate or poly(hydroxybutyrate-co-hydroxyvalerate). Ind. Crop. Prod. 2017, 99, 117–125. [Google Scholar] [CrossRef]
- Uzun, G.; Aydemir, D. Biocomposites from polyhydroxybutyrate and bio-fillers by solvent casting method. Bull. Mater. Sci. 2017, 40, 383–393. [Google Scholar] [CrossRef]
- Liu, H.Z.; Zhang, J.W. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jimenez, A. Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stabil. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(l-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Lopez, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohyd. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Castro-Lopez, M.D.; Rayon, E.; Barral-Losada, L.F.; Lopez-Vilarino, J.M.; Lopez, J.; Gonzalez-Rodriguez, M.V. Plasticized poly(lactic acid)-poly(hydroxybutyrate) (PLA-PHB) blends incorporated with catechin intended for active food-packaging applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl citrate (TEC) as a dispersing aid in polylactic acid/chitin nanocomposites prepared via liquid-assisted extrusion. Polymers 2017, 9, 406. [Google Scholar] [CrossRef]
- Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(l-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178–7188. [Google Scholar] [CrossRef]
- Sungsanit, K.; Kao, N.; Bhattacharya, S.N. Properties of linear poly(lactic acid)/polyethylene glycol blends. Polym. Eng. Sci. 2012, 52, 108–116. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/poly(ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef]
- Jing, Z.; Shi, X.; Zhang, G. Competitive stereocomplexation and homocrystallization behaviors in the poly(lactide) blends of PLLA and PDLA-PEG-PDLA with controlled block length. Polymers 2017, 9, 107. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized jatropha oil as a sustainable plasticizer to poly(lactic acid). Polymers 2017, 9, 204. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Samper, M.D.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid). Ind. Crop. Prod. 2017, 104, 278–286. [Google Scholar] [CrossRef]
- Ferri, J.M.; Samper, M.D.; Garcia-Sanoguera, D.; Reig, M.J.; Fenollar, O.; Balart, R. Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly(lactic acid). J. Mater. Sci. 2016, 51, 5356–5366. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Montanes, N.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil as biobased plasticizer in poly(lactic acid)-based formulations. Polym. Int. 2017, 66, 882–891. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K. Advances in the properties of polylactides based materials: A review. J. Biobased Mater. Bioenergy 2007, 1, 191–209. [Google Scholar] [CrossRef]
- Supthanyakul, R.; Kaabbuathong, N.; Chirachanchai, S. Poly(l-lactide-b-butylene succinate-b-l-lactide) triblock copolymer: A multi-functional additive for PLA/PBS blend with a key performance on film clarity. Polym. Degrad. Stabil. 2017, 142, 160–168. [Google Scholar] [CrossRef]
- Wu, L.P.; Chen, S.T.; Li, Z.B.; Xu, K.T.; Chen, G.Q. Synthesis, characterization and biocompatibility of novel biodegradable poly((R)-3-hydroxybutyrate)-block-(d,l-lactide)-block-(epsilon-caprolac tone) triblock copolymers. Polym. Int. 2008, 57, 939–949. [Google Scholar] [CrossRef]
- Carrasco, F.; Cailloux, J.; Sanchez-Jimenez, P.E.; Maspoch, M.L. Improvement of the thermal stability of branched poly(lactic acid) obtained by reactive extrusion. Polym. Degrad. Stabil. 2014, 104, 40–49. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; Garcia-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jimenez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crop. Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Barmbalexis, P.; Bikiaris, D.N. Novel electrospun nanofibrous matrices prepared frompoly(lactic acid)/poly(butylene adipate) blends for controlled release formulations of an anti-rheumatoid agent. Eur. J. Pharm. Sci. 2016, 88, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Zolali, A.M.; Heshmati, V.; Favis, B.D. Ultratough co-continuous PLA/PA11 by interfacially percolated poly(ether-b-amide). Macromolecules 2017, 50, 264–274. [Google Scholar] [CrossRef]
- García-Campo, M.J.; Quiles-Carrillo, L.; Masia, J.; Reig-Pérez, M.J.; Montanes, N.; Balart, R. Environmentally friendly compatibilizers from soybean oil for ternary blends of poly(lactic acid)-PLA, poly(ε-caprolactone)-PCL and poly(3-hydroxybutyrate)-PHB. Materials 2017, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; Akhtar, T.; Luckachan, G.; Matsko, N. PLA, TPS and PCL binary and ternary blends: Structural characterization and time-dependent morphological changes. Colloid Polym. Sci. 2015, 293, 573–585. [Google Scholar] [CrossRef]
- Xu, Y.W.; Loi, J.; Delgado, P.; Topolkaraev, V.; McEneany, R.J.; Macosko, C.W.; Hillmyer, M.A. Reactive compatibilization of polylactide/polypropylene blends. Ind. Eng. Chem. Res. 2015, 54, 6108–6114. [Google Scholar] [CrossRef]
- Yang, S.Z.; Madbouly, S.A.; Schrader, J.A.; Srinivasan, G.; Grewell, D.; McCabe, K.G.; Kessler, M.R.; Graves, W.R. Characterization and biodegradation behavior of bio-based poly(lactic acid) and soy protein blends for sustainable horticultural applications. Green Chem. 2015, 17, 380–393. [Google Scholar] [CrossRef]
- Hachemi, R.; Belhaneche-Bensemra, N.; Massardier, V. Elaboration and characterization of bioblends based on PVC/PLA. J. Appl. Polym. Sci. 2014, 131, 7. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Ovyn, R.; Malinconico, M.; Rubino, P.; Grohens, Y. Peculiar crystallization kinetics of biodegradable poly(lactic acid)/poly(propylene carbonate) blends. Polym. Eng. Sci. 2015, 55, 2698–2705. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Biopolymer blends based on poly(lactic acid): Shear and elongation rheology/structure/blowing process relationships. Polymers 2015, 7, 939–962. [Google Scholar] [CrossRef]
- Si, P.; Luo, F.; Luo, F. Miscibility, morphology and crystallization behavior of poly(butylene succinate-co-butylene adipate)/poly(vinyl phenol)/poly(l-lactic acid) blends. Polymers 2016, 8, 421. [Google Scholar] [CrossRef]
- Pradeep, S.A.; Kharbas, H.; Turng, L.-S.; Avalos, A.; Lawrence, J.G.; Pilla, S. Investigation of thermal and thermomechanical properties of biodegradable PLA/PBSA composites processed via supercritical fluid-assisted foam injection molding. Polymers 2017, 9, 22. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Sustainable biobased blends of poly(lactic acid) (PLA) and poly(glycerol succinate-co-maleate) (PGSMA) with balanced performance prepared by dynamic vulcanization. RSC Adv. 2017, 7, 38594–38603. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, B.; Bian, X.C.; Li, G.; Chen, X.S. High melt strength and high toughness PLLA/PBS blends by copolymerization and in situ reactive compatibilization. Ind. Eng. Chem. Res. 2017, 56, 52–62. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Taylor, S.; Misra, M.; Mohanty, A.K. Thermo-mechanical characterization of bioblends from polylactide and poly(butylene adipate-co-terephthalate) and lignin. Macromol. Mater. Eng. 2015, 300, 299–311. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Poly(lactic acid) toughening with a better balance of properties. Macromol. Mater. Eng. 2010, 295, 204–209. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Mittal, V. Evaluation of crystallinity variation and phase dispersion in polymer blends and nanocomposites by raman mapping. J. Polym. Res. 2015, 22, 12. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lopez, J.; Hernandez, A.; Rayon, E. Ternary PLA-PHB-limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stabil. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Rizzuto, M.; Marinetti, L.; Caretti, D.; Mugica, A.; Zubitur, M.; Mueller, A.J. Can poly(epsilon-caprolactone) crystals nucleate glassy polylactide? Crystengcomm 2017, 19, 3178–3191. [Google Scholar] [CrossRef]
- Kelnar, I.; Kratochvil, J.; Kapralkova, L. Crystallization and thermal properties of melt-drawn PCL/PLA microfibrillar composites. J. Therm. Anal. Calorim. 2016, 124, 799–805. [Google Scholar] [CrossRef]
- Yeh, J.-T.; Wu, C.-J.; Tsou, C.-H.; Chai, W.-L.; Chow, J.-D.; Huang, C.-Y.; Chen, K.-N.; Wu, C.-S. Study on the crystallization, miscibility, morphology, properties of poly(lactic acid)/poly(-caprolactone) blends. Polym. Plast. Technol. Eng. 2009, 48, 571–578. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xiong, C.D.; Deng, X.M. Miscibility, crystallization and morphology of poly(beta-hydroxybutyrate)/poly(d,l-lactide) blends. Polymer 1996, 37, 235–241. [Google Scholar] [CrossRef]
- Chen, C.C.; Chueh, J.Y.; Tseng, H.; Huang, H.M.; Lee, S.Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly(R,S-3-hydroxy butyrate)-morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Gerard, T.; Budtova, T.; Podshivalov, A.; Bronnikov, S. Polylactide/poly(hydroxybutyrate-co-hydroxyvalerate) blends: Morphology and mechanical properties. Express Polym. Lett. 2014, 8, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Socrate, S. Micromechanics of uniaxial tensile deformation and failure in high impact polystyrene (HIPS). Polymer 2009, 50, 3386–3395. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Montes, M.L.I.; Manfredi, L.B.; Cyras, V.P. Fully bio-based and biodegradable polylactic acid/poly(3-hydroxybutirate) blends: Use of a common plasticizer as performance improvement strategy. Polym. Test 2016, 49, 22–28. [Google Scholar] [CrossRef]
- Patricio, T.; Bartolo, P. Thermal stability of PCL/PLA blends produced by physical blending process. In 3rd International Conference on Tissue Engineering; Bartolo, P., Fernandes, P., Eds.; Elsevier Science Bv: Amsterdam, The Netherlands, 2013; Volume 59, pp. 292–297. [Google Scholar]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly(lactic acid) (PLA)/poly(epsilon-caprolactone) (PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. Polym. Test 2015, 45, 93–100. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lopez, J.; Lopez, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA-PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. Miscibility study of PHB and PLA mixtures, using a PHB with high polydispersity. Polimeros 2015, 25, 76–82. [Google Scholar]
- Lim, J.S.; Park, K.I.; Chung, G.S.; Kim, J.H. Effect of composition ratio on the thermal and physical properties of semicrystalline PLA/PHB-HHx composites. Mat. Sci. Eng. C Mater. 2013, 33, 2131–2137. [Google Scholar] [CrossRef] [PubMed]







| Code | PLA (wt %) | PHB (wt %) | PCL (wt %) |
|---|---|---|---|
| 100/0/0 | 100 | 0 | 0 |
| 60/40/0 | 60 | 40 | 0 |
| 60/30/10 | 60 | 30 | 10 |
| 60/20/20 | 60 | 20 | 20 |
| 60/10/30 | 60 | 10 | 30 |
| 60/0/40 | 60 | 0 | 40 |
| PLA/PHB/PCL (wt %) | Impact-Absorbed Energy (kJ m−2) | Shore D Hardness |
|---|---|---|
| 100/0/0 | 1.63 ± 0.14 | 69.0 ± 1.2 |
| 60/40/0 | 1.79 ± 0.10 | 69.6 ± 0.9 |
| 60/30/10 | 2.40 ± 0.38 | 69.3 ± 0.7 |
| 60/20/20 | 2.45 ± 0.62 | 67.0 ± 0.9 |
| 60/10/30 | 5.06 ± 0.94 | 65.0 ± 1.0 |
| 60/0/40 | 6.13 ± 0.21 | 65.2 ± 0.3 |
| PLA/PHB/PCL (wt %) | T5 (°C) | Tmax (°C) | Residual Weight (%) | ||
|---|---|---|---|---|---|
| PHB | PLA | PCL | |||
| 100/0/0 | 328.5 ± 2.6 | - | 368.5 ± 0.9 | - | 0.36 ± 0.12 |
| 60/40/0 | 281.8 ± 2.9 | 291.5 ± 1.2 | 363.2 ± 0.7 | 390.6 ± 1.2 | 4.49 ± 0.24 |
| 60/30/10 | 284.8 ± 3.3 | 292.5 ± 0.9 | 363.8 ± 0.8 | 403.2 ± 1.6 | 4.23 ± 0.23 |
| 60/20/20 | 285.5 ± 3.4 | 291.8 ± 1.1 | 363.6 ± 0.8 | 408.1 ± 1.3 | 2.73 ± 0.41 |
| 60/10/30 | 294.3 ± 3.1 | 297.4 ± 0.6 | 360.6 ± 1.1 | 407.4 ± 0.9 | 1.82 ± 0.54 |
| 60/0/40 | 336.0 ± 2.4 | - | 366.3 ± 0.6 | 413.3 ± 0.7 | 2.54 ± 0.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Campo, M.J.; Boronat, T.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Manufacturing and Characterization of Toughened Poly(lactic acid) (PLA) Formulations by Ternary Blends with Biopolyesters. Polymers 2018, 10, 3. https://doi.org/10.3390/polym10010003
García-Campo MJ, Boronat T, Quiles-Carrillo L, Balart R, Montanes N. Manufacturing and Characterization of Toughened Poly(lactic acid) (PLA) Formulations by Ternary Blends with Biopolyesters. Polymers. 2018; 10(1):3. https://doi.org/10.3390/polym10010003
Chicago/Turabian StyleGarcía-Campo, María Jesús, Teodomiro Boronat, Luis Quiles-Carrillo, Rafael Balart, and Nestor Montanes. 2018. "Manufacturing and Characterization of Toughened Poly(lactic acid) (PLA) Formulations by Ternary Blends with Biopolyesters" Polymers 10, no. 1: 3. https://doi.org/10.3390/polym10010003
APA StyleGarcía-Campo, M. J., Boronat, T., Quiles-Carrillo, L., Balart, R., & Montanes, N. (2018). Manufacturing and Characterization of Toughened Poly(lactic acid) (PLA) Formulations by Ternary Blends with Biopolyesters. Polymers, 10(1), 3. https://doi.org/10.3390/polym10010003