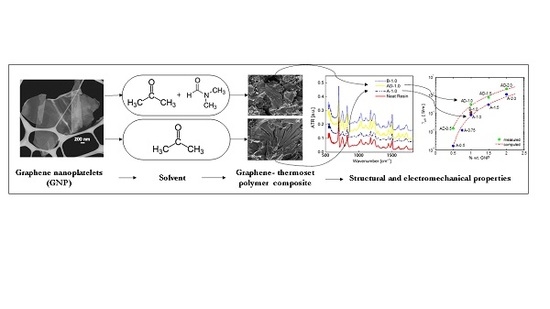
Electrical, Mechanical and Electromechanical Properties of Graphene-Thermoset Polymer Composites Produced Using Acetone-DMF Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. GNP Production and Composite Fabrication
2.2. Morphological Investigation
2.3. Fourier Transform Infrared Spectrometer
2.4. Dynamic Mechanical Thermal Analysis
2.5. Electrical Conductivity Measurement
2.6. Mechanical and Electromechanical Tensile Tests
3. Results
3.1. Morphology
3.2. Fourier Transform Infrared Spectrometer
3.3. Dynamic Mechanical Thermal Analysis
3.4. Mechanical Properties
3.5. Electrical Properties
3.6. Electromechanical Response
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Kumar, A.; Chouhan, D.K.; Alegaonkar, P.S.; Patro, T.U. Graphene-like nanocarbon: An effective nanofiller for improving the mechanical and thermal properties of polymer at low weight fractions. Compos. Sci. Technol. 2016, 127, 79–87. [Google Scholar] [CrossRef]
- Hu, N.; Karube, Y.; Yan, C.; Masuda, Z.; Fukunaga, H. Tunneling effect in a polymer/carbon nanotube nanocomposite strain sensor. Acta Mater. 2008, 56, 2929–2936. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.H.; Dang, Z.M.; Jiang, M.J.; Xu, H.P.; Bai, J. Influence of aspect ratio of carbon nanotube on percolation threshold in ferroelectric polymer nanocomposite. Appl. Phys. Lett. 2007, 91, 212901. [Google Scholar] [CrossRef]
- Zhu, Z.H. Piezoresistive Strain Sensors Based on Carbon Nanotube Networks: Contemporary approaches related to electrical conductivity. IEEE Nanotechnol. Mag. 2015, 9, 11–23. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- D’Aloia, A.G.; Marra, F.; Tamburrano, A.; De Bellis, G.; Sarto, M.S. Electromagnetic absorbing properties of graphene-polymer composite shields. Carbon 2014, 73, 175–184. [Google Scholar] [CrossRef]
- Sarto, M.S.; D’Aloia, A.G.; Tamburrano, A.; De Bellis, G. Synthesis, modeling, and experimental characterization of graphite nanoplatelet-based composites for EMC applications. IEEE Trans. Electromagn. Compat. 2012, 54, 17–27. [Google Scholar] [CrossRef]
- De Bellis, G.; Tamburrano, A.; Dinescu, A.; Santarelli, M.L.; Sarto, M.S. Electromagnetic properties of composites containing graphite nanoplatelets at radio frequency. Carbon 2011, 49, 4291–4300. [Google Scholar] [CrossRef]
- Yan, D.X.; Pang, H.; Li, B.; Vajtai, R.; Xu, L.; Ren, P.-G.; Wang, J.H.; Li, Z.-M. Structured Reduced Graphene Oxide/Polymer Composites for Ultra-Efficient Electromagnetic Interference Shielding. Adv. Funct. Mater. 2015, 25, 559–566. [Google Scholar] [CrossRef]
- Verma, M.; Verma, P.; Dhawan, S.K.; Choudhary, V. Tailored graphene based polyurethane composites for efficient electrostatic dissipation and electromagnetic interference shielding applications. RSC Adv. 2015, 5, 97349–97358. [Google Scholar] [CrossRef]
- Tjong, S.C. Polymer composites with graphene nanofillers: Electrical properties and applications. J. Nanosci. Nanotechnol. 2014, 14, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Koul, S.; Chandra, R.; Dhawan, S.K. Conducting polyaniline composite for ESD and EMI at 101 GHz. Polymer 2000, 41, 9305–9310. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Park, H.W.; Park, Y.-B. Piezoresistive behavior and multi-directional strain sensing ability of carbon nanotube–graphene nanoplatelet hybrid sheets. Smart Mater. Struct. 2013, 22, 015013. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Shi, Z.; Zhang, L.; Shi, D.; Wang, E.; Zhang, G. Super-elastic graphene ripples for flexible strain sensors. ACS Nano 2011, 5, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Tamburrano, A.; Fortunato, M.; Sarto, M.S. A Flexible and Highly Sensitive Pressure Sensor Based on a PDMS Foam Coated with Graphene Nanoplatelets. Sensors 2016, 16, 2148. [Google Scholar] [CrossRef] [PubMed]
- Acquarelli, C.; Paliotta, L.; Tamburrano, A.; De Bellis, G.; Sarto, M.S. Electro-Mechanical Properties of Multilayer Graphene-Based Polymeric Composite Obtained through a Capillary Rise Method. Sensors 2016, 16, 1780. [Google Scholar] [CrossRef] [PubMed]
- Tamburrano, A.; Sarasini, F.; De Bellis, G.; D’Aloia, A.G.; Sarto, M.S. The piezoresistive effect in graphene-based polymeric composites. Nanotechnology 2013, 24, 465702. [Google Scholar] [CrossRef] [PubMed]
- Bidsorkhi, H.C.; D’Aloia, A.G.; De Bellis, G.; Proietti, A.; Rinaldi, A.; Fortunato, M.; Ballirano, P.; Bracciale, M.P.; Santarelli, M.L.; Sarto, M.S. Nucleation effect of unmodified graphene nanoplatelets on PVDF/GNP film composites. Mater. Today Commun. 2017, 11, 163–173. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Wang, C.; Yuan, D.; Stubbs, L.P.; He, C. The Effect of Residual Solvent N,N′-Dimethylformamide on the Curing Reaction and Mechanical Properties of Epoxy and Lignin Epoxy Composites. Macromol. Chem. Phys. 2016, 217, 1065–1073. [Google Scholar] [CrossRef]
- Tang, C.; Li, X.; Li, Z.; Hao, J. Interfacial Hydrogen Bonds and Their Influence Mechanism on Increasing the Thermal Stability of Nano-SiO2-Modified Meta-Aramid Fibres. Polymers 2017, 9, 504. [Google Scholar] [CrossRef]
- Mondragon, I.; Bucknall, C.B. Effects of residual dichloromethane solvent on the cure of epoxy resin. Plast. Rubber Compos. Process. Appl. 1994, 21, 275–281. [Google Scholar]
- Lau, K.; Lu, M.; Lam, C.; Cheung, H.; Sheng, F.; Li, H. Thermal and mechanical properties of single-walled carbon nanotube bundle-reinforced epoxy nanocomposites: The role of solvent for nanotube dispersion. Compos. Sci. Technol. 2005, 65, 719–725. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.; Lopes, A.C.; Botelho, G.; Neves, I.C.; Lanceros-Mendez, S. Influence of solvent properties on the electrical response of poly(vinylidene fluoride)/NaY composites. J. Polym. Res. 2013, 20, 143. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Tensile Properties of Plastics; ASTM D638; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Lewis, T.B.; Nielsen, L.E. Dynamic mechanical properties of particulate-filled composites. J. Appl. Polym. Sci. 2013, 14, 1449–1471. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, Z.; Liu, L.; Yin, Z. Modelling hydrogen bonds in N,N-dimethylformamide. Mol. Simul. 2013, 39, 875–881. [Google Scholar] [CrossRef]
- McManis, G.E. Infrared absorption spectra of vinyl esters of carboxylic acids. Appl. Spectrosc. 1970, 24, 495–498. [Google Scholar] [CrossRef]
- Shundalau, M.B.; Chybirai, P.S.; Komyak, A.I.; Zazhogin, A.P.; Ksenofontov, M.A.; Umreiko, D.S. Modeling of structures and calculation of IR vibrational spectra of N,N-dimethylformamide dimers by density functional theory. J. Appl. Spectrosc. 2011, 78, 326–336. [Google Scholar] [CrossRef]
- Jacob, M.M.E.; Arof, A.K. FTIR studies of DMF plasticized polyvinyledene fluoride based polymer electrolytes. Electrochim. Acta 2000, 45, 1701–1706. [Google Scholar] [CrossRef]
- George, M.H. Polymerization of styrene in N,N-dimethylformamide and N,N-dimethylacetamide. J. Polym. Sci. A 1964, 2, 3169–3178. [Google Scholar] [CrossRef]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Rahimi, M.; Müller-Plathe, F. Molecular dynamics simulation of a silica nanoparticle in oligomeric poly(methyl methacrylate): A model system for studying the interphase thickness in a polymer-nanocomposite via different properties. Macromolecules 2013, 46, 8680–8692. [Google Scholar] [CrossRef]
- Riccardi, E.; Böhm, M.C.; Müller-Plathe, F. Molecular dynamics method to locally resolve Poisson’s ratio: Mechanical description of the solid-soft-matter interphase. Phys. Rev. E 2012, 86, 036704. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, N.; Bagheri, S. Molecular dynamics simulation of nanoconfined n-decane. J. Mol. Liq. 2013, 180, 101–106. [Google Scholar] [CrossRef]
- Staufer, D.; Aharony, A. Introduction to Percolation Theory; Tailor & Francis: London, UK, 1985. [Google Scholar]
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.; Ferreira, M.S.; Möbius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science 2016, 354, 1257–1260. [Google Scholar] [CrossRef] [PubMed]













| Sample name | GNP (wt %) * | Solvent | |
|---|---|---|---|
| Acetone (vol %) ** | DMF (vol %) ** | ||
| A-0.5 | 0.50 | 100 | 0 |
| A-0.75 | 0.75 | 100 | 0 |
| A-1.0 | 1.00 | 100 | 0 |
| A-1.5 | 1.50 | 100 | 0 |
| A-2.0 | 2.00 | 100 | 0 |
| AD-0.5 | 0.50 | 90 | 10 |
| AD-1.0 | 1.00 | 90 | 10 |
| AD-1.5 | 1.50 | 90 | 10 |
| AD-2.0 | 2.00 | 90 | 10 |
| B-1.0 | 1.0 | 97 | 3 |
| Sample | Young’s modulus (GPa) |
|---|---|
| Neat resin | 3.25 ± 0.03 |
| A-1.0 | 3.54 ± 0.04 |
| A-1.5 | 3.50 ± 0.06 |
| A-2.0 | 2.87 ± 0.14 |
| AD-1.0 | 1.87 ± 0.09 |
| AD-1.5 | 0.47 ± 0.15 |
| AD-2.0 | 0.31 ± 0.11 |
| B-1.0 | 3.20 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aloia, A.G.; Proietti, A.; Bidsorkhi, H.C.; Tamburrano, A.; De Bellis, G.; Marra, F.; Bregnocchi, A.; Sarto, M.S. Electrical, Mechanical and Electromechanical Properties of Graphene-Thermoset Polymer Composites Produced Using Acetone-DMF Solvents. Polymers 2018, 10, 82. https://doi.org/10.3390/polym10010082
D’Aloia AG, Proietti A, Bidsorkhi HC, Tamburrano A, De Bellis G, Marra F, Bregnocchi A, Sarto MS. Electrical, Mechanical and Electromechanical Properties of Graphene-Thermoset Polymer Composites Produced Using Acetone-DMF Solvents. Polymers. 2018; 10(1):82. https://doi.org/10.3390/polym10010082
Chicago/Turabian StyleD’Aloia, Alessandro Giuseppe, Alessandro Proietti, Hossein Cheraghi Bidsorkhi, Alessio Tamburrano, Giovanni De Bellis, Fabrizio Marra, Agnese Bregnocchi, and Maria Sabrina Sarto. 2018. "Electrical, Mechanical and Electromechanical Properties of Graphene-Thermoset Polymer Composites Produced Using Acetone-DMF Solvents" Polymers 10, no. 1: 82. https://doi.org/10.3390/polym10010082
APA StyleD’Aloia, A. G., Proietti, A., Bidsorkhi, H. C., Tamburrano, A., De Bellis, G., Marra, F., Bregnocchi, A., & Sarto, M. S. (2018). Electrical, Mechanical and Electromechanical Properties of Graphene-Thermoset Polymer Composites Produced Using Acetone-DMF Solvents. Polymers, 10(1), 82. https://doi.org/10.3390/polym10010082