The formation of the capsules may be affected by many variables resulting from equipment construction and the physicochemical properties of encapsulated substances and media for their immobilization, such as: viscosity, surface tension of the core and shell solution, and the interactions between them.
The polymer capsules were created using the drop-in-drop coaxial method. For the gelation of the chitosan and sodium alginate capsules, alcoholic solutions of sodium hydroxide and calcium lactate were used, respectively. Sodium hydroxide causes the fall out of the chitosan from the solution as a microcrystalline precipitate, while the presence of ethyl alcohol significantly accelerates this process [
28]. This composition of the solution allows avoiding oil leakage from the capsule after falling into the hardening solution. The fast sodium alginate gelation reaction occurs in the presence of calcium ions, creating a molecular structure called the egg-box model [
29]. An additional advantage of the lactate form of the calcium salt is the lack of influence on taste.
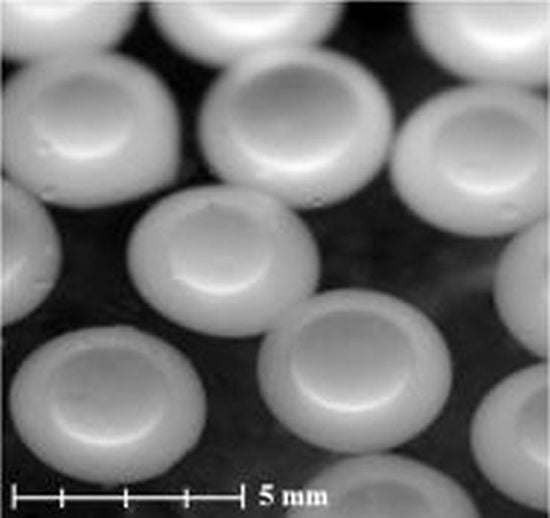
The particle size distribution measurement showed that the chitosan capsules’ diameter was in the range of 3.9 ÷ 4.3 mm, where the capsules with diameters of 3.9, 4.0, 4.1, 4.2, and 4.3 mm represented 1.4 ± 0.2%, 3.4 ± 0.4%, 8.0 ± 1.0%, 83.7 ± 3.4%, and 4.1 ± 0.2% of the studied population, respectively. This range is consistent with the results that we obtained in our earlier work [
10]. In the case of capsules with a sodium alginate shell, the diameter range was 3.7 ÷ 4.1 mm. The alginate capsules with diameters of 3.7, 3.8, 3.9, 4.0, and 4.1 mm represented 0.9 ± 0.1%, 4.5 ± 0.1%, 15.7 ± 1.6%, 78.7 ± 4.0%, and 1.8 ± 0.2% of the studied population, respectively.
Relative to the review work of Bakry et al. [
8], this is a value higher than that obtained during encapsulation by emulsification and interfacial polymerization, and also comparable with the encapsulation efficiency using extrusion, spray drying, or fluidized bed-coating methods. The hydrogel capsules contained 38–45% oil, where the chitosan capsules were characterized by a higher oil content in the core of about 10% compared to alginate capsules. This is the small, but significant difference that can be related to the viscosity and surface tension of the shell polymer solutions [
30]. A 2% chitosan solution has a higher viscosity than a 2% solution of sodium alginate, which may indicate that the first one is less susceptible to shear forces associated with dispensing oil into the shell solution droplets, and is more efficient at filling with the same encapsulation efficiency.
3.1. Impact of Encapsulation Process on the Oil Quality
The research material was the refined rapeseed oil “Kujawski”, having at least one year’s shelf-life, and the cold-pressed, high-linoleic linseed oil “Wielkopolski”, for which the shelf-life date was at least half a year. In all of the tests, homogeneous batches of rapeseed oil and linseed oil were obtained by combining and mixing five liters of oil from smaller unit packages. The prepared oil samples were encapsulated using the coaxial method according to
Section 2.3 [
10].
The quality of each oil (rapeseed and linseed, encapsulated and before encapsulation) has been examined based on the peroxide, anisidine, and acid values.
Figure 1 shows that the encapsulation did not significantly affect the content of the primary oxidation products in rapeseed oil. In linseed oil, the peroxide value increased by approximately 20% relative to the oil sample prior to encapsulation. Nevertheless, the peroxide values of linseed oil before and after the process were lower compared to the corresponding samples of rapeseed oil (
Figure 1a).
In addition to the undesirable substances, many substances with antioxidant properties (e.g., tocopherols, carotenoids, sterols) were removed from the oil during the refining process, which could be the reason for this situation [
31]. In all of the tests, the peroxide value did not exceed the maximum allowable limit of five mEq O
2/kg for refined rapeseed oil [
32] and linseed oil as cold-pressed fat equal to 15 mEq O
2/kg oil [
33]. All of the tested oil samples were characterized by a low and similar anisidine value (
Figure 1b). Makareviciene and Janulis [
34] stated that the level of this value for refined oils should not exceed eight units. According to the same authors, the maximum AV for virgin oils should not exceed three units, while Subramanian et al. [
35] claimed that the upper limit of the
p-anisidinenumber has not been determined so far exclusively for cold-pressed oils, and should be not higher than two units. In none of the tested samples was the limit exceeded (
Figure 1b). It can be concluded that the encapsulation did not result in a statistically significant change in the content of secondary products of the two tested oils, irrespective of the type of polymer used to create the capsule shell. The encapsulation also did not change the content of free fatty acids in the analyzed oils. Both before and after encapsulation, these values were the same for oils of the same type (
Figure 1c).
The permissible value of the free fatty acid has not been exceeded for either rapeseed oil samples: 0.3 mg KOH/kg of oil [
32] and for linseed oil samples: 4.0 mg KOH/kg of fat, for which the quality requirements are identical with the quality requirements for cold-pressed vegetable oils from various raw materials [
33]. Higher values of the acid number of linseed oil than rapeseed oil can be explained by the rapeseed oil refining step, during which free fatty acids are effectively removed from the oil [
36].
3.2. Protective Role of Encapsulation on the Oil Quality during Storage
The rapeseed oil and linseed oil that were prepared for determination in accordance with the procedure described in
Section 3.1 were used to carry out the experiment. The prepared oil samples were encapsulated using the coaxial method according to
Section 2.3. The quality of oil (rapeseed and linseed, encapsulated and non-encapsulated) after two and four weeks storage has been examined based on the peroxide, anisidine, and acid values, as well as the fatty acid profile.
The PV of rapeseed oil and linseed oil in the control samples and encapsulated samples before the start of the test was small (
Table 2) and comparable to the values measured immediately after encapsulation in the previous test (
Figure 1a).
The highest PV of linseed oil was obtained after four weeks of storage for the non-encapsulated sample. Moreover, it was the highest value of all of the samples determined after this time. For the linseed oil sample stored under the same conditions in the form of alginate capsules, the result was 20% lower. The greatest protective effect in the test was obtained by encapsulating the linseed oil in chitosan hydrogel. In this case, the PV was 88% lower than the value obtained for the control sample (
Table 2). It was the only sample in which the permissible value of the peroxide number determining the possibility of oil consumption was not exceeded. The highest PV of rapeseed oil was obtained in the control sample after four weeks of storage. The content of peroxides in the rapeseed oil that was stored for four weeks in the form of chitosan and alginate capsules was 40% lower than that in the control sample (
Table 2).
The analyzed samples showed different levels of secondary oxidation products (SOPs) depending on the type of oil and its storage form. In each of the samples, the anisidine value increased during the storage time. The highest AV after the end of the test was obtained for control samples, rapeseed oil, and linseed oil (
Table 3).
In turn, the lowest value was obtained for linseed oil stored in the form of chitosan capsules, and rapeseed oil stored in the form of alginate capsules (
Table 3). In the case of the linseed oil encapsulated in sodium alginate, the SOPs content was approximately 20% higher than in the oil inside the chitosan shell. In the case of rapeseed oil, encapsulation in the chitosan polymer caused the generation of only 5% more SOPs than in the alginate one.
The content of free fatty acids (FFAs) in rapeseed oil was low in the control and encapsulated samples. After four weeks of storage at 40 °C, the FFAs’ content increased equally, regardless of the oil form that was used in the test (
Table 4). This means that the encapsulation stage does not contribute to the formation of free fatty acids. What’s more, the AcV values after the test fulfilled the requirements [
32], according to which the permissible value for refined rapeseed oil does not exceed 0.3 mg KOH/kg. The quality requirements for cold-pressed linseed oil are identical to the quality requirements for cold-pressed vegetable oils from various raw materials: 4.0 mg KOH/kg [
33]. The content of FFAs in non-encapsulated and encapsulated linseed oil was similar during the test and after it was finished (
Table 4). The free fatty acids are removed during refining oil at the deacidification stage in the form of soaps; hence, their higher content in linseed oil is fully justified [
36].
In both oils, the total content of saturated fatty acids (SFAs) was in the range of 8–10%. Both oils were also characterized by a high and similar share of unsaturated acids, with 60–65% content of monounsaturated fatty acids (MUFAs) and 25–30% content of polyunsaturated acids (PUFAs) in rapeseed oil. In the case of linseed oil, the content of MUFAs and PUFAs was exactly the opposite of the amounts that were measured for the rapeseed oil (
Table 5 and
Table 6).
The dominant fatty acids in rapeseed and linseed oil were oleic acid and linolenic acid, respectively. The content of other acids was typical for the raw materials from which they were obtained, and consistent with the data available in the scientific literature [
37,
38].
Table 5 and
Table 6 present the changes in the composition of fatty acids that occurred in rapeseed and linseed oil during storage in unencapsulated form and in the form of chitosan and alginate capsules.
The results indicate that statistically significant changes in the fatty acids profile in rapeseed oil occurred only in the case of the non-encapsulated form of oil. These changes consisted in an increase of the SFAs content (10%), which was mainly palmitic and stearic acid, and the decrease in the content of MUFAs and PUFAs (each 5%). In the samples that were stored for four weeks at 40 °C in encapsulated form, no significant changes were observed in the fatty acids profile. The content of trans fatty acids listed in the table also did not change (
Table 5).
A similar relationship regarding the change of the fatty acids profile was observed in the case of linseed oil. The statistically significant changes concerned an increase in the content of SFAs (7.5%) and a decrease in the content of MUFAs (3.5%) and PUFAs (1.3%). In samples stored for four weeks at 40 °C in encapsulated form, no significant changes were observed in the fatty acids profile. The content of trans fatty acids listed in the table had also not changed (
Table 6). Fatty acids composition is one of the most important factors that determine the oxidative stability of the oils. It is known that the rate of C18:2 acid oxidation is 10–40 times higher than that of C18:1, and the rate of C18:3 oxidation is two to four times faster than that of C18:2 [
39]. According to this relationship, changes in the composition of fatty acids should be more noticeable in the case of linseed oil.
In a study of White et al. [
40], the composition of linseed oil changed only slightly during storage over six months. Only the increase in the amount of palmitic acid was statistically significant, e.g., from 6.6% to 7.0% in variety Linola
TM 947. The above results are in confirmation with the findings of Ayton et al. [
41], who reported a slight increase of SFAs and decrease of PUFAs up to 1.3%, in an olive oil sample. The content of individual fatty acids did not change significantly only in the samples encapsulated regarding the control oils, which partly confirms to a certain extent the protective function of the hydrogel membrane made of the chitosan or sodium alginate. However, the measurement of changes in the composition of fatty acids can not be in this case a clear indicator of the capsules’ protective role. The data that are available in the scientific literature regarding changes in the fatty acids profile during the storage of edible oils are not entirely predictable. They are the resultant of factors, such as fatty acids composition, time and temperature of storage, the presence of different compounds in oil affecting the rate of radical generation, or the type of food in which the oil was present. For example, changes that occurred in the composition of fatty acids in frozen calves’ meat stored for three months, due to the increase of PUFAs level, are inconsistent with the mechanism of fat autooxidation [
42].
Both oils before encapsulation were characterized by a similar content of tocopherols, which was expressed as the sum of these compounds (
Table 7).
The content of tocopherols in rapeseed and linseed oil before the start of the test is comparable with the data contained in other works [
43,
44,
45]. After the test, non-encapsulated rapeseed oil was characterized by a 44% lower content of total tocopherols, of which 37% was the reduction of the form alpha. In the rapeseed oil encapsulated in the chitosan and alginate membranes, the content of tocopherols decreased by 5% and 16%, respectively (
Table 7). In the case of linseed oil, the reduction of total tocopherol content for the control, and CHI and ALG samples, was 21%, 7%, and 11%, respectively. It was found that the singlet oxygen oxidation rate of tocopherols varied in the order α > γ > δ [
46], which is also confirmed by our results. Goffman and Möllers [
47], in research on the stability of tocopherols in rapeseed oil, showed that individual tocopherols do not degrade in 5 °C and 20 °C during 24 weeks of storage. However, at 40 °C, degradation was already observed after four to six weeks.
Wagner and Elmadfa [
48] showed the stabilizing effect of tocopherols and their mixtures (100 mg/100 g oil) in linseed oil: γ-T > γ/δ-T > δ-T > γ/α-T > α-T> α/δ-T. So, the greater protective effect of encapsulation observed in the case of linseed oil may be the result of a synergistic action of two factors. The first is the protective role of polymers in the form of hydrogel shells acting as a physical barrier limiting oxygen access to the inside of capsules, where the solubility of oxygen in membrane is similar to water, and about 10 times lower than in edible oil (3 mol/L in oil) [
49]. The second is the higher content of a more stable γ-tocopherol form in linseed oil (over 90% of total tocopherol value).
Tańska et al. [
50] showed a high positive correlation between peroxide value and dienes content (>0.82) in rapeseed and linseed oil, which means that the largest increase in dienes should be in oil samples without encapsulation. The obtained results confirm the above correlation (
Table 8).
The content of conjugated dienes and trienes in the samples of linseed and rapeseed oil before storage and after the test in the form of capsules did not statistically differ, and was comparable to result obtained in similar works (
Table 5) [
31,
50,
51]. From the statistical point of view, the obtained results may indicate the slower unfavorable changes of encapsulated oil, especially linseed oil with a high content of linolenic acid.