New Horizons in Cationic Photopolymerization
Abstract
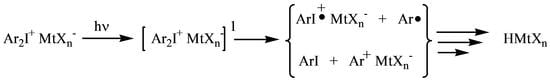
:1. Introduction
2. New Cationic Photoinitiators
3. Frontal Polymerization
4. Conclusions
Conflicts of Interest
References
- Sangermano, M. UV Cured nanostructured epoxy coatings. In Epoxy Polymers New Materials and Innovations; Pascault, J.P., Williams, R.J.J., Eds.; Wiley: Weinheim, Germany, 2010; pp. 235–249. [Google Scholar]
- Dufour, P. Radiation Curing in Polymer Science and Technology—Vol I: Fundamentals and Methods; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier Science Publishers: London, UK; New York, NY, USA, 1993; pp. 1–28. [Google Scholar]
- Sangermano, M.; Chiolerio, A. Silver and Gold polymer nanocomposites and electrical properties thereof. In Nanoparticles Featuring Properties: From Science to Engineering; Chiolerio, A., Allia, P., Eds.; Research Signpost: Kerala, India, 2012; pp. 85–104. [Google Scholar]
- Vitale, A.; Sangermano, M.; Bongiovanni, R.; Burtscher, P.; Moszner, N. Visible light curable restorative composites for dental applications based on epoxy monomer. Materials 2014, 7, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Sturari, M.; Chiappone, A.; Roppolo, I. Study of Ink-Jet Printable Vinyl Ether-Graphene UV-Curable Formulations. Macromol. Mater. Eng. 2015, 300, 340–345. [Google Scholar] [CrossRef]
- Gonzalez, G.; Chiappone, A.; Roppolo, I.; Fantino, E.; Bertana, V.; Perrucci, F.; Scaltrito, L.; Pirri, F.; Sangermano, M. Development of 3D printable formulations containing CNT with enhanced electrical properties. Polymer 2017, 109, 246–253. [Google Scholar] [CrossRef]
- Chiappone, A.; Roppolo, I.; Naretto, E.; Fantino, E.; Calignano, F.; Sangermano, M.; Pirri, F. Study of graphene oxide-based 3D printable composites: Effect of the in situ reduction. Compos. Part B Eng. 2017, 124, 9–15. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1316. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Dye-sensitized photoinitiated cationic polymerization. J. Polym. Sci. Part A Polym. Chem. 1978, 16, 2441–2451. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Photoinitiated cationic polymerization with triarylsulfonium salts. J. Polym. Sci. Part A Polym. Chem. 1979, 17, 977–999. [Google Scholar] [CrossRef]
- Crivello, J.V.; Ma, J.; Jiang, F. Synthesis and photoactivity of novel 5-arylthianthrenium salt cationic photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3465–3480. [Google Scholar] [CrossRef]
- Crivello, J.V. The discovery and development of onium salt cationic photoinitiators. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 4241–4254. [Google Scholar] [CrossRef]
- Sangermano, M. Advances in cationic photopolymerization. Pure Appl. Chem. 2012, 84, 2089–2101. [Google Scholar] [CrossRef]
- Crivello, J.V.; Dietliker, K. Photoinitiators for Free Radical, Cationic and Anionic Polymerization, 2nd ed.; Wiley: New York, NY, USA, 1998; p. 479. [Google Scholar]
- Crivello, J.V.; Reichmamis, E. Photopolymer materials and processes for advanced technologies. Chem. Mater. 2014, 26, 533–548. [Google Scholar] [CrossRef]
- Pappas, S.P.; Gatechair, L.R.; Jilek, J.H. Photoinitiation of cationic polymerization. IV. Direct and sensitized photolysis of aryl iodonium and sulfonium salts. Polym. Photochem. 1984, 5, 1–22. [Google Scholar] [CrossRef]
- Yagci, Y.; Reetz, I. Externally stimulated initiator systems for cationic polymerization. Prog. Polym. Sci. 1998, 23, 1485–1538. [Google Scholar] [CrossRef]
- Davidson, S. Exploring the Science, Technology and Applications of UV and EB Curing; SITA: London, UK, 1999. [Google Scholar]
- Decker, C. Kinetic study and new applications of UV radiation curing. Macromol. Rapid Commun. 2002, 23, 1067–1093. [Google Scholar] [CrossRef]
- Crivello, J.V. Cationic polymerization: Iodonium and sulfonium photoinitiators. Adv. Polym. Sci. 1984, 62, 2–23. [Google Scholar]
- Shia, S.; Croutxé-Barghorna, C.; Allonas, X. Photoinitiating systems for cationic photopolymerization: Ongoing push toward long wavelengths and low light intensities. Prog. Polym. Sci. 2017, 65, 1–41. [Google Scholar] [CrossRef]
- Yagci, Y.; Kornowski, A.; Schnabel, W. N-alkoxy-pyridinium and N-alkoxy-quinolinium salts as initiators for cationic photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1987–1991. [Google Scholar]
- Yagci, Y.; Endo, T. N-benzyl and N-alkoxy pyridinium salts as thermal and photochemical initiators for cationic polymerization. Adv. Polym. Sci. 1997, 127, 59–86. [Google Scholar]
- Yagci, Y. Photoinitiated cationic polymerization of unconventional monomers. Macromol. Symp. 2006, 240, 93–101. [Google Scholar] [CrossRef]
- Yagci, Y.; Jovkusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Burr, D.; Crivello, J.V. Photochemistry and photopolymerization activity of diaryliodonium salts. J. Macromol. Sci. Pure Appl. Chem. 1994, A31, 677–701. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. Photosensitized cationic polymerizations using dialkylphenacylsulfonium and dialkyl (4-hydroxyphenyl) sulfonium salt photoinitiators. Macromolecules 1981, 14, 1141–1147. [Google Scholar] [CrossRef]
- Nelson, E.W.; Carter, T.P.; Scranton, A.B. The role of the triplet state in the photosensitization of cationic polymerizations by anthracene. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 247–256. [Google Scholar] [CrossRef]
- Yagci, Y.; Schnabel, W.; Wilpert, A.; Bendig, J. Electron transfer from aromatic compounds to phenyliodinium and diphenylsulfinium radical cations. J. Chem. Soc. Faraday Trans. 1994, 90, 287–291. [Google Scholar] [CrossRef]
- Hizal, G.; Yagci, Y.; Schnabel, W. Charge-transfer complexes of pyridinium ions and methyl-and methoxy-substituted benzenes as photoinitiators for the cationic polymerization of cyclohexene oxide and related compounds. Polymer 1994, 35, 2428–2431. [Google Scholar] [CrossRef]
- Hizal, G.; Emiroglu, S.E.; Yagci, Y. Photoinitiated radical polymerization using charge transfer complex of N-ethoxy-p-cyanopyridinium salt and 1,2,4-trimethoxybenzene. Polym. Int. 1998, 47, 391–392. [Google Scholar] [CrossRef]
- Schnabel, W. Cationic photopolymerization with the aid of pyridinium-type salts. Macrmoml. Rapid Commun. 2000, 21, 628–642. [Google Scholar] [CrossRef]
- Denisligil, S.; Yagci, Y.; McArdel, C. Photochemically and thermally induced radical promoted cationic polymerization using an allylic sulfonium salt. Polymer 1995, 36, 3093–3098. [Google Scholar] [CrossRef]
- Yagci, Y.; Schnabel, W. New aspects on the photoinitiated free radical promoted cationic polymerization. In Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1992; Volume 60, pp. 133–143. [Google Scholar]
- Crivello, J.V. A new visible light sensitive photoinitiator system for the cationic polymerization of epoxides. J. Polym. Sci. Part A Polym. Chem. 2009, 866–875. [Google Scholar] [CrossRef]
- Cook, W.D.; Chen, S.; Chen, F.; Khreci, M.U.; Yagci, Y. Photopolymerization of vinyl ether networks using an iodonium initiator. The role of photosensitizers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5474–5487. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Gaff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Variations on the benzophenone skeleton: Novel high performance blue light sensitive photoinitiating systems. Macromolecules 2013, 46, 7661–7667. [Google Scholar] [CrossRef]
- Xiaoa, P.; Zhanga, J.; Dumur, F.; Ali Tehfea, M.; Morlet-Savarya, F.; Graffa, B.; Gigmesb, D.; Fouassierc, J.P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Crivello, J.V.; Sangermano, M. Visible and long wavelength photoinitiated cationic polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 343–356. [Google Scholar] [CrossRef]
- Klikovits, N.; Knaack, P.; Bomze, D.; Krossing, I.; Liska, R. Novel photoacid generators for cationic photopolymerization. Polym. Chem. 2017, 8, 4414–4421. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Visible-light-sensitive photoredox catalysis by iron complexes: Applications in cationic and radical polymerization reactions. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2247–2253. [Google Scholar] [CrossRef]
- Crivello, J.V.; Liu, S. Free radical induced acceleration of cationic photopolymerization. Chem. Mater. 1998, 10, 3724–3731. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Horcajada, P.; Livage, C.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Iron-Based Metal-Organic Frameworks (MOF) as Photocatalysts for Radical and Cationic Polymerizations under Near UV and Visible LEDs (385–405 nm). Macromol. Chem. Phys. 2016, 217, 2534–2540. [Google Scholar] [CrossRef]
- Lecompère, M.; Allonas, X.; Maréchal, D.; Criqui, A. Dual-cure Photo-thermal Initiating System for Cationic Polymerization of Epoxy under LED Visible Light. J. Photopolym. Sci. Technol. 2017, 30, 399–404. [Google Scholar] [CrossRef]
- Kwon, S.; Chun, H.; Mah, S. Photo-induced living cationic polymerization of isobutyl vinyl ether in the presence of various combinations of halides of diphenyliodonium and zinc salts in methylene chloride. Fibers Polym. 2004, 5, 253–258. [Google Scholar] [CrossRef]
- Pojman, J.A. Traveling fronts of methacrylic acid polymerization. J. Am. Chem. Soc. 1991, 113, 6284–6286. [Google Scholar] [CrossRef]
- Pojman, J.A.; Elcan, W.; Khan, A.M.; Mathias, L. Binary frontal polymerization: A new method to produce simultaneous interpenetrating polymer networks (SINs). J. Polym. Sci. Part A Polym. Chem. 1997, 35, 227–230. [Google Scholar] [CrossRef]
- Crivello, J.V.; Rajaraman, S.; Mowers, W.A.; Liu, S. Free radical accelerated cationic polymerizations. Macromol. Symp. 2000, 157, 109–120. [Google Scholar] [CrossRef]
- Ledwith, A. Possibility for promoting cationic polymerization by common sources of free radicals. Polymer 1978, 19, 1217–1227. [Google Scholar] [CrossRef]
- Mariani, A.; Bidali, S.; Fiori, S.; Sangermano, M.; Malucelli, G.; Bongiovanni, R.; Priola, A. UV-ignited frontal polymerization of an epoxy resin. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2066–2072. [Google Scholar] [CrossRef]
- Bomze, D.; Knaack, P.; Liska, R. Successful radical induced cationic frontal polymerization of epoxy-based monomers by C-C labile compounds. Polym. Chem. 2015, 6, 8161–8167. [Google Scholar] [CrossRef]
- Klikovits, N.; Liska, R.; D’Anna, A.; Sangermano, M. Successful UV-Induced RICFP of Epoxy-Composites. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]




| Organic linker | Terephthalic acid | Fumaric acid | Terephthalic acid | Azobenzene tetracarboxylic acid | Trimesic acid |
|---|---|---|---|---|---|
 |  |  |  |  | |
| Cristalline structure |  |  |  |  |  |
| Chemical formula | Fe(OH)[C8O4H4] nsolv | Fe3O(OH)(H2O)2 [C4O4H2]3 nsolv | Fe3O(OH)(H2O)2 [C8O4H4]3 nsolv | Fe3O(OH)(H2O)2 [C16O8N2H6]2 nsolv | Fe3O(OH)(H2O)2 [C9O6H3]2 nsolv |
| %Fe | 23.6 (chains) | 30.9 (trimers) | 24.2 (trimers) | 21.4 (trimers) | 25.8 (trimers) |
| Flexibility | Yes | Yes | Yes | No | No |
| Pore size (Å) | 8.5 | 6.5 | 9 | 6 10 | 25 (5) 19 (8.6) |
| Particle size (µm) | 2–3 | 5–9 | 0.06–0.10 | 0.5–0.7 | 0.1–0.4 0.05–0.10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. https://doi.org/10.3390/polym10020136
Sangermano M, Roppolo I, Chiappone A. New Horizons in Cationic Photopolymerization. Polymers. 2018; 10(2):136. https://doi.org/10.3390/polym10020136
Chicago/Turabian StyleSangermano, Marco, Ignazio Roppolo, and Annalisa Chiappone. 2018. "New Horizons in Cationic Photopolymerization" Polymers 10, no. 2: 136. https://doi.org/10.3390/polym10020136
APA StyleSangermano, M., Roppolo, I., & Chiappone, A. (2018). New Horizons in Cationic Photopolymerization. Polymers, 10(2), 136. https://doi.org/10.3390/polym10020136