Preparation of 2-Aminothiazole-Functionalized Poly(glycidyl methacrylate) Microspheres and Their Excellent Gold Ion Adsorption Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
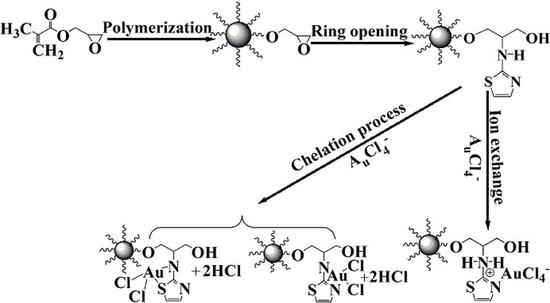
2.2. Synthesis of A-PGMA Adsorbent
2.3. Adsorption Experiments
2.4. Characterization
3. Results and Discussion
3.1. Characterization
3.2. Adsorption Behavior of Gold on A-PGMA
3.2.1. Effect of pH on Gold Adsorption
3.2.2. Effect of Contact Time and Adsorption Kinetics
3.2.3. Effect of Original Gold Concentration and Adsorption Isotherms
3.2.4. Reusability
3.2.5. Selectivity
3.2.6. Adsorption Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forno, I.; Grande, M.A.; Fais, A. On the application of Electro-sinter-forging to the sintering of high-karatage gold powders. Gold Bull. 2015, 48, 127–133. [Google Scholar] [CrossRef]
- Barbieri, L.; Giovanardi, R.; Lancellotti, I.; Michelazzi, M. A new environmentally friendly process for the recovery of gold from electronic waste. Environ. Chem. Lett. 2010, 8, 171–178. [Google Scholar] [CrossRef]
- Konował, E.; Sikorska, A.M.; Motylenko, M.; Klapiszewski, Ł.; Wysokowski, M.; Bazhenov, V.V.; Rafaja, D.; Ehrlich, H.; Milczarek, G.; Jesionowski, T. Functionalization of organically modified silica with gold nanoparticles in the presence of lignosulfonate. Int. J. Biol. Macromol. 2016, 85, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Konował, E.; Sikorska, A.M.; Milczarek, G. Synthesis and multifunctional properties of lignosulfonate-stabilized gold nanoparticles. Mater. Lett. 2015, 159, 451–454. [Google Scholar] [CrossRef]
- Roslan, N.A.; Suah, F.B.M.; Mohamed, N. The use of an electrogenerative process as a greener method for recovery of gold(III) from the E-waste. Sep. Purif. Technol. 2017, 182, 1–8. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Won, S.W.; Kotte, P.; Wei, W.; Lim, A.; Yun, Y.S. Biosorbents for recovery of precious metals. Bioresour. Technol. 2014, 160, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Mortaheb, H.R.; Zolfaghari, A.; Mokhtarani, B.; Amini, M.H.; Mandanipour, V. Study on removal of cadmium by hybrid liquid membrane process. J. Hazard. Mater. 2010, 177, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Nishihama, S.; Yoshizuka, K. Separation and recovery of gold from waste LED using ion exchange method. Hydrometallurgy 2015, 157, 194–198. [Google Scholar] [CrossRef]
- Mulwanda, J.; Dorfling, C. Recovery of dissolved platinum group metals from copper sulphate leach solutions by precipitation. Miner. Eng. 2015, 80, 50–56. [Google Scholar] [CrossRef]
- Mohammadi, T.; Moheb, A.; Sadrzadeh, M.; Razmi, A. Modeling of metal ion removal from wastewater by electrodialysis. Sep. Purif. Technol. 2005, 41, 73–82. [Google Scholar] [CrossRef]
- Shah, K.; Gupta, K.; Sengupta, B. Selective separation of copper and zinc from spent chloride brass pickle liquors using solvent extraction and metal recovery by precipitation-stripping. J. Environ. Chem. Eng. 2017, 5, 5260–5269. [Google Scholar] [CrossRef]
- Fan, R.; Xie, F.; Guan, X.; Zhang, Q.; Luo, Z. Selective adsorption and recovery of Au(III) from three kinds of acidic systems by persimmon residual based bio-sorbent: A method for gold recycling from e-wastes. Bioresour. Technol. 2014, 163, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Yanuar, E. Leaching and Adsorption of Gold from Lape-Sumbawa Rocks (Indonesia) by Hypochlorite-Chloride. Procedia Chem. 2015, 17, 59–65. [Google Scholar] [CrossRef]
- Yong, S.O.; Jeon, C. Selective adsorption of the gold–cyanide complex from waste rinse water using Dowex 21K XLT resin. J. Ind. Eng. Chem. 2014, 20, 1308–1312. [Google Scholar]
- Sun, C.; Zhang, G.; Wang, C.; Qu, R.; Zhang, Y.; Gu, Q. A resin with high adsorption selectivity for Au(III): Preparation, characterization and adsorption properties. Chem. Eng. J. 2011, 172, 713–720. [Google Scholar]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Turki, A.; Guillard, C.; Dappozze, F.; Ksibi, Z.; Berhault, G.; Kochkar, H. Phenol photocatalytic degradation over anisotropic TiO2 nanomaterials: Kinetic study, adsorption isotherms and formal mechanisms. Appl. Catal. B Environ. 2015, 163, 404–414. [Google Scholar] [CrossRef]
- Najafi, M.; Yousefi, Y.; Rafati, A.A. Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Albishri, H.M.; Marwani, H.M. Chemically modified activated carbon with tris(hydroxymethyl)aminomethane for selective adsorption and determination of gold in water samples. Arab. J. Chem. 2016, 9, 252–258. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Xing, J. Selective adsorption behavior of Cd(II) ion imprinted polymers synthesized by microwave-assisted inverse emulsion polymerization: Adsorption performance and mechanism. J. Hazard. Mater. 2017, 321, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, Y.; Liu, T.; Wu, W. Recovery of uranium(VI) from aqueous solution by 2-picolylamine functionalized poly(styrene-co-maleic anhydride) resin. J. Colloid Interface Sci. 2017, 497, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dong, J.; Li, J.; Ye, M.; Zhang, W.; Ou, J. Facile preparation of polysaccharide functionalized macroporous adsorption resin for highly selective enrichment of glycopeptides. J. Chromatogr. A 2017, 1498, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, R. Extended study of DETA-functionalized PGMA adsorbent in the selective adsorption behaviors and mechanisms for heavy metal ions of Cu, Co, Ni, Zn, and Cd. J. Colloid Interface Sci. 2010, 350, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Hou, C.; He, X.; Liu, M. Preparation of a novel TETA functionalized magnetic PGMA nano-absorbent by ATRP method and used for highly effective adsorption of Hg(II). J. Taiwan Inst. Chem. Eng. 2015, 58, 283–289. [Google Scholar] [CrossRef]
- Horák, D.; Shapoval, P. Reactive poly(glycidyl methacrylate) microspheres prepared by dispersion polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3855–3863. [Google Scholar] [CrossRef]
- Ghaedi, A.M.; Ghaedi, M.; Vafaei, A.; Iravani, N.; Keshavarz, M.; Rad, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Adsorption of copper(II) using modified activated carbon prepared from Pomegranate wood: Optimization by bee algorithm and response surface methodology. J. Mol. Liq. 2015, 206, 195–206. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhao, R.; Lu, X.; Xu, X.; Jia, X.; Wang, C.; Li, L. Efficient adsorption of gold ions from aqueous systems with thioamide-group chelating nanofiber membranes. Chem. Eng. J. 2013, 229, 420–428. [Google Scholar] [CrossRef]
- Bai, L.; Hu, H.; Fu, W.; Wan, J.; Cheng, X.; Zhuge, L.; Xiong, L.; Chen, Q. Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J. Hazard. Mater. 2011, 195, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Repo, E.; Kurniawan, T.A.; Warchol, J.K.; Sillanpää, M.E.T. Removal of Co(II) and Ni(II) ions from contaminated water using silica gel functionalized with EDTA and/or DTPA as chelating agents. J. Hazard. Mater. 2009, 171, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, P.; Liu, X.; Dong, X.; Zhang, J.; Xu, Q. Thermodynamics, kinetics, and isotherms studies for gold(III) adsorption using silica functionalized by diethylenetriaminemethylenephosphonic acid. Chem. Eng. Res. Des. 2013, 91, 2748–2758. [Google Scholar] [CrossRef]
- He, Z.W.; He, L.H.; Yang, J.; Lü, Q.F. Removal and recovery of Au(III) from aqueous solution using a low-cost lignin-based biosorbent. Ind. Eng. Chem. Res. 2013, 52, 4103–4108. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Cao, L.; Zhang, X.; Yang, C. Gold-recovery PVDF membrane functionalized with thiosemicarbazide. Chem. Eng. J. 2015, 280, 399–408. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Radhakrishnan, P.G. Thermodynamics and kinetics of adsorption of Cu(II) from aqueous solutions onto a new cation exchanger derived from tamarind fruit shell. J. Chem. Thermodyn. 2008, 40, 702–709. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical Models of Sorption Kinetics Including a Surface Reaction Mechanism: A Review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Da’Na, E.; Silva, N.D.; Sayari, A. Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Kinetics properties. Chem. Eng. J. 2011, 166, 454–459. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Kuang, S.P.; Wang, Z.Z.; Liu, J.; Wu, Z.C. Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb(II) ion from aqueous solutions. J. Hazard. Mater. 2013, 260, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Sun, W.Y.; Guo, N.; Ding, S.L.; Su, S.J. Comparison of Co2+ adsorption by chitosan and its triethylene-tetramine derivative: Performance and mechanism. Carbohydr. Polym. 2016, 151, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.C.; Paul, D.; Borse, A.U.; Garole, D.J. Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep. Purif. Technol. 2018, 195, 260–270. [Google Scholar] [CrossRef]
- Khosravi, R.; Azizi, A.; Ghaedrahmati, R.; Gupta, V.K.; Agarwal, S. Adsorption of gold from cyanide leaching solution onto activated carbon originating from coconut shell—Optimization, kinetics and equilibrium studies. J. Ind. Eng. Chem. 2017, 54, 464–471. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Bandaru, N.M.; Reta, N.; Dalal, H.; Ellis, A.V.; Shapter, J.; Voelcker, N.H. Enhanced adsorption of mercury ions on thiol derivatized single wall carbon nanotubes. J. Hazard. Mater. 2013, 261, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Can, N.; Ömür, B.C.; Altındal, A. Modeling of Heavy Metal Ion Adsorption Isotherms onto Metallophthalocyanine Film. Sens. Actuators B Chem. 2016, 237, 953–961. [Google Scholar] [CrossRef]
- Ertan, E.; Gülfen, M. Separation of gold(III) ions from copper(II) and zinc(II) ions using thiourea–formaldehyde or urea–formaldehyde chelating resins. J. Appl. Polym. Sci. 2009, 111, 2798–2805. [Google Scholar] [CrossRef]
- Chen, X.; Lam, K.F.; Mak, S.F.; Yeung, K.L. Precious metal recovery by selective adsorption using biosorbents. J. Hazard. Mater. 2011, 186, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Aydın, A.; İmamoğlu, M.; Gülfen, M. Separation and recovery of gold(III) from base metal ions using melamine–formaldehyde–thiourea chelating resin. J. Appl. Polym. Sci. 2008, 107, 1201–1206. [Google Scholar] [CrossRef]
- Koyanaka, H.; Takeuchi, K.; Loong, C.K. Gold recovery from parts-per-trillion-level aqueous solutions by a nano-structured Mn2O3 adsorbent. Sep. Purif. Technol. 2005, 43, 9–15. [Google Scholar] [CrossRef]
- Pang, S.K.; Yung, K.C. Prerequisites for achieving gold adsorption by multiwalled carbon nanotubes in gold recovery. Chem. Eng. Sci. 2014, 107, 58–65. [Google Scholar] [CrossRef]
- Kondratowicz, I.; Żelechowska, K.; Nadolska, M.; Jażdżewska, A.; Gazda, M. Comprehensive study on graphene hydrogels and aerogels synthesis and their ability of gold nanoparticles adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2017, 528, 65–73. [Google Scholar] [CrossRef]
- Tsekouras, G.; Ralph, S.F.; Price, W.E.; Wallace, G.G. Gold recovery using inherently conducting polymer coated textiles. Fibers Polym. 2004, 5, 1–5. [Google Scholar] [CrossRef]
- Payne, R.; Magee, R.J.; Liesegang, J. (II) Infrared and X-ray photoelectron spectroscopy of some transition metal dithiocarbamates and xanthates. J. Electron. Spectrosc. Relat. Phenom. 1985, 35, 113–130. [Google Scholar] [CrossRef]

















| Kinetics Models | Parameters |
|---|---|
| Pseudo-first-order | k1 = 0.1458 min−1 |
| R2 = 0.6973 qe = 181.47 | |
| Pseudo-second-order | k2 = 0.00107 g/(mg·min) |
| R2 = 0.9746 qe = 196.28 | |
| Intraparticle diffusion | k3 = 4.3583 |
| R2 = 0.7799 |
| Stage | k3 | R2 | C |
|---|---|---|---|
| I | 11.4769 | 0.97895 | 85.62776 |
| II | 5.04205 | 0.99949 | 125.4287 |
| III | 0.32316 | −0.10323 | 189.948 |
| Isotherms Models | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Parameters | qmax | KL | R2 | KF | n | R2 |
| Values | 440.84 | 0.4352 | 0.9911 | 338.53 | 22.237 | 0.9389 |
| Adsorbents | qm (mg/g) | Reference |
|---|---|---|
| Activated carbon | 3.54 | [45] |
| Thiourea–formaldehyde resin | 21.56 | [46] |
| Silk sericin and chitosan biosorbents | 37.00 | [47] |
| Cellulose | 34.40 | [48] |
| Melamine–formaldehyde–thiourea chelating resin | 48.00 | [49] |
| Nano-structured Mn2O3 | 70.0 | [50] |
| Multiwalled carbon nanotubes | 93.5 | [51] |
| Graphene hydrogels | 103.50 | [52] |
| Nylon-lycra or PPy | 115.00 | [53] |
| A-PGMA | 440.54 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, C.; Wang, S.; Zhang, L.; Li, Y.; Zhou, Y.; Peng, J. Preparation of 2-Aminothiazole-Functionalized Poly(glycidyl methacrylate) Microspheres and Their Excellent Gold Ion Adsorption Properties. Polymers 2018, 10, 159. https://doi.org/10.3390/polym10020159
Xiong C, Wang S, Zhang L, Li Y, Zhou Y, Peng J. Preparation of 2-Aminothiazole-Functionalized Poly(glycidyl methacrylate) Microspheres and Their Excellent Gold Ion Adsorption Properties. Polymers. 2018; 10(2):159. https://doi.org/10.3390/polym10020159
Chicago/Turabian StyleXiong, Chao, Shixing Wang, Libo Zhang, Ying Li, Yang Zhou, and Jinhui Peng. 2018. "Preparation of 2-Aminothiazole-Functionalized Poly(glycidyl methacrylate) Microspheres and Their Excellent Gold Ion Adsorption Properties" Polymers 10, no. 2: 159. https://doi.org/10.3390/polym10020159
APA StyleXiong, C., Wang, S., Zhang, L., Li, Y., Zhou, Y., & Peng, J. (2018). Preparation of 2-Aminothiazole-Functionalized Poly(glycidyl methacrylate) Microspheres and Their Excellent Gold Ion Adsorption Properties. Polymers, 10(2), 159. https://doi.org/10.3390/polym10020159