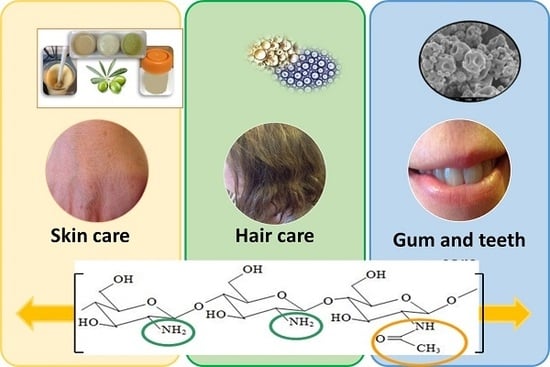
Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives
Abstract
:1. Introduction
2. Target Organs for Cosmetics Products
2.1. Gums
2.2. Teeth
2.3. Hair
2.4. Skin
3. Chitin, Chitosan and their Derivatives in Cosmetics and Cosmeceutical Industry
3.1. Chitosan and Derivatives in Oral Healthcare
3.1.1. Reduction of Dental Plaque
3.1.2. Reduction of Dental Abrasion
3.1.3. Chitosan as Vehicle in Oral Healthcare
3.2. Chitin, Chitosan and Derivatives in Haircare
3.2.1. Chitosan and Derivatives as Hair Care Ingredient
3.2.2. Chitosan as a Vehicle in Hair Care
3.3. Chitin, Chitosan and Their Derivatives in Skin Care
3.3.1. Application of Chitin, Chitosan and its Derivatives in UV Protection
3.3.2. Use as Skin Cleansing, Skin Conditioning and Emollient
3.3.3. Use as Humectant and Moisturizing Agent
3.3.4. Use as Surfactant, Emulsifier, Stabilizer and Viscosifier
3.3.5. Use as Antioxidant and Antimicrobial Agent
3.3.6. Chitosan and Derivatives as Vehicle for Active Ingredients in Skin Care
4. Functional Characterization of Chitin and Chitosan in Cosmetics
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- European Commission. Glossary and Acronyms Related to Cosmetics Legislation; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Reed, E. The definition of “cosmeceuticals”. J. Soc. Cosm. Chem. 1962, 13, 103–110. [Google Scholar]
- Morganti, P. Reflections on cosmetics, cosmeceuticals, and nutraceuticals. Clin. Dermatol. 2008, 26, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Sud, M. Cosmeceuticals. Clin. Dermatol. 2008, 26, 317. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-H.; Zhang, L.; Wei, H.; Chen, H.-D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, B.J.; Gilchrest, B.A.; Vermeer; Friedel, S.L. Cosmeceuticals: A proposal for rational definition, evaluation, and regulation. Arch. Dermatol. 1996, 132, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. Why cosmeceuticals? Cosmet. Toilet. 1993, 108, 37–38. [Google Scholar]
- Saint-Leger, D. “Cosmeceuticals”. Of men, science and laws.. Int. J. Cosmet. Sci. 2012, 34, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Wijesekara, I. Cosmeceuticals from marine resources. Prospects and commercial trends. In Marine Cosmeceuticals. Trends and Prospects; Kim, S.K., Ed.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2012; pp. 1–9. [Google Scholar]
- Senevirathne, W.S.M.; Kim, S.K. 23-Cosmeceuticals from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 694–713. ISBN 978-0-85709-512-1. [Google Scholar]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chapter 25—Chitin and Chitosan: Major Sources, Properties and Applications A2—Belgacem, Mohamed Naceur. In Monomers, Polymers and Composites from Renewable Resources; Gandini, A., Ed.; Elsevier: Amsterdam, The Netherland, 2008; pp. 517–542. ISBN 978-0-08-045316-3. [Google Scholar]
- Kinney, J.S.; Morelli, T.; Braun, T.; Ramseie, C.A.; Herr, A.E.; Sugai, J.V.; Shelburne, C.E.; Rayburn, L.A.; Singh, A.K.; Giannobile, W.V. Saliva/Pathogen Biomarker Signatures and Periodontal Disease Progression. J. Dent. Res. 2011, 90, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Dhadse, P.; Gattani, D.; Mishra, R. The link between periodontal disease and cardiovascular disease: How far we have come in last two decades ? J. Indian Soc. Periodontol. 2010, 14, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, J.Y.; Youk, T.M.; Jeong, S.N.; Kim, Y.T.; Choi, S.H. Association between periodontal disease and non-communicable diseases: A 12-year longitudinal health-examinee cohort study in South Korea. Medicine 2017, 96, e7398. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O.; Kidd, E.A.M.; Nyvad, B.; Baelum, V. Defining the disease: An introduction. In Dental Caries. The Disease and Its Clinical Management, 2nd ed.; Fejerskov, O., Kidd, E., Eds.; Blackwell Munksgaard Ltd.: Oxford, UK, 2003. [Google Scholar]
- Imfeld, T. Dental erosion Definition, classification and links. Eur. J. Oral Sci. 1996, 104, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.E. Body Surface Area. In A Dictionary of Food and Nutrition; DA, B., Ed.; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Shimizu, H. Chapter 1: Structure and Function of the Skin. In Shimizu’s Textbook of Dermatology; Nakayama Shoten Publishers; Hokkaido University Press: Hokkaido, Japan, 2007. [Google Scholar]
- Agrawal, S.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Marine fungi: An untapped bioresource for future cosmeceuticals. Phytochem. Lett. 2018, 23, 15–20. [Google Scholar] [CrossRef]
- Acosta, N.; Sánchez, E.; Calderón, L.; Cordoba-Diaz, M.; Cordoba-Diaz, D.; Dom, S.; Heras, Á. Physical Stability Studies of Semi-Solid Formulations from Natural Compounds Loaded with Chitosan Microspheres. Mar. Drugs 2015, 13, 5901–5919. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of on cosmetic products. Off. J. Eur. Union 2009, 1223, 342–359. [Google Scholar]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.W.; Grychowska, N.; Paradowska-Stolarz, A. Clinical Application of Chitosan in Dental Specialities. Mini-Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Sano, H.; Matsukubo, T.; Takaesu, Y. The influences of the buffer capacity of various substances on pH changes in dental plaque. Bull. Tokyo Dent. Coll. 1994, 35, 27–32. [Google Scholar] [PubMed]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Shibasaki, K.; Sano, H.; Matsukubo, T.; Takaesu, Y. Effects of low molecular chitosan on pH changes in human dental plaque. Bull. Tokyo Dent. Coll. 1994, 35, 33–39. [Google Scholar] [PubMed]
- Ji, Q.X.; Zhong, D.Y.; Lv, R.; Zhang, W.Q.; Deng, J.; Chen, X.G. In vitro evaluation of the biomedical properties of chitosan and quaternized chitosan for dental applications. Carbohydr. Res. 2009. [Google Scholar] [CrossRef] [PubMed]
- İkinci, G.; Şenel, S.; Akıncıbay, H.; Kaş, S.; Erciş, S.; Wilson, C.G.; Hıncal, A.A. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int. J. Pharm. 2002, 235, 121–127. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Veiga, M.; Tavaria, F.K.; Manuela, M. A review of chitosan´s effect on oral bio films : Perspectives from the tube to the mouth. J. Oral Biosci. 2017, 59, 1–6. [Google Scholar] [CrossRef]
- Aliasghari, A.; Rabbani Khorasgani, M.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran J. Microbiol. 2016, 8, 93–100. [Google Scholar] [PubMed]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, R.; Maturana, C.; Silva, D.; Tobar, N.; Tapia, C.; Salazar, J.C.; Martínez, J.; Smith, P.C. Effects of Chitosan Particles in Periodontal Pathogens and Gingival Fibroblasts. J. Dent. Res. 2013, 92, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Tarsi, R.; Corbin, B.; Pruzzo, C.; Muzzarelli, R.A. Effect of low-molecular-weight chitosans on the adhesive properties of oral streptococci. Oral Microbiol. Immunol. 1998, 217–224. [Google Scholar] [CrossRef]
- Ganguly, A.; Ian, C.K.; Sheshala, R.; Sahu, P.S.; Al-Waeli, H.; Meka, V.S. Application of diverse natural polymers in the design of oral gels for the treatment of periodontal diseases. J. Mater. Sci. Mater. Med. 2017, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Arancibia, R.; Tapia, C.; Acuña-Rougier, C.; Diaz-Dosque, M.; Cáceres, M.; Martínez, J.; Smith, P.C. Chitosan and platelet-derived growth factor synergistically stimulate cell proliferation in gingival fibroblasts. J. Periodontal Res. 2013, 48, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tarsi, R.; Muzzarelli, R.A.; Guzmán, C.A.; Pruzzo, C. Inhibition of Streptococcus mutans adsorption to hydroxyapatite by low-molecular-weight chitosans. J. Dent. Res. 1997, 76, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Study of the effects of chitosan upon Streptococcus mutans adherence and biofilm formation. Anaerobe 2013, 20, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Shibasaki, K.; Matsukubo, T.; Takaesu, Y. Effect of molecular mass and degree of deacetylation of chitosan on adsorption of Streptococcus sobrinus 6715 to saliva treated hydroxyapatite. Bull. Tokyo Dent. Coll. 2002, 43, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Achmad, H.; Ramadhany, Y.F. Effectiveness of chitosan tooth paste from white shrimp (Litopenaeus vannamei) to reduce number of Streptococcus mutans in the case of early childhood Caries. J. Int. Dent. Med. Res. 2017, 10, 358–363. [Google Scholar]
- Sano, H.; Shibasaki, K.-I.; Matsukubo, T.; Takaesu, Y. Effect of chitosan rinsing or reduction of dental plaque formation. Bull. Tokyo Dent. Coll. J. 2003. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Madureira, A.R.; Cardelle-Cobas, A.; Tavaria, F.K.; Pintado, M.M. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr. Polym. 2014, 101, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Costa, M.R.; Pereira, M.; Campos, D.A.; Odila, J.; Madureira, A.R.; Cardelle-Cobas, A.; Tavaria, F.K.; Rodrigues, A.S.; Pintado, M.M. Chitosan mouthwash: Toxicity and in vivo validation. Carbohydr. Polym. 2014, 111, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Emilson, C.G. Susceptibility of various microorganisms to chlorhexidine. Scand. J. Dent. Res. 1977, 85, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.M.; von Ohle, C.; Weiger, R.; Wiech, I.; Brecx, M. A synergistic chlorhexidine/chitosan combination for improved antiplaque strategies. J. Periodontal Res. 2005, 40, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Mohire, N.C.; Yadav, A. V Chitosan-based polyherbal toothpaste: As novel oral hygiene product. Indian J. Dent. Res. 2010, 21, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Ohara, N.; Ganno, T.; Yamaguchi, K.; Ishizaki, T.; Nakamura, T.; Sato, M. Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch. Oral Biol. 2007, 52, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Ohara, N.; Ganno, T.; Ishizaki, H.; Yanagiguchi, K. Chitosan-containing gum chewing accelerates antibacterial effect with an increase in salivary secretion. J. Dent. 2007, 35, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Matsukubo, T.; Shibasaki, K.; Itoi, H.; Takaesu, Y. Inhibition of adsorption of oral streptococci to saliva treated hydroxyapatite by chitin derivatives. Bull. Tokyo Dent. Coll. 1991, 32, 9–17. [Google Scholar] [PubMed]
- Sano, H.; Shibasaki, K.; Matsukubo, T.; Takaesu, Y. Effect of rinsing with phosphorylated chitosan on four-day plaque regrowth. Bull. Tokyo Dent. Coll. 2001, 42, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.U.; Chung, Y.-C. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. J. Appl. Oral Sci. 2012, 20, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jun, E.J.; Lee, S.M.; Paik, D.I.; Kim, J.B. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin. Oral Invest. 2006, 10, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Schulze, K.; Schlueter, N. Toothpaste and Erosion. In Toothpastes. Monogr Oral Sci. Basel; van Loveren, C., Ed.; Karger: Amsterdam, The Netherland, 2013; Volume 23, pp. 88–99. [Google Scholar]
- Ozalp, S.; Tulunoglu, O. SEM–EDX analysis of brushing abrasion of chitosan and propolis based toothpastes on sound and artificial carious primary enamel surfaces. Int. J. Paediatr. Dent. 2014, 24, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Klimek, J.; Ganss, C. Effect of a chitosan additive to a Sn2+-containing toothpaste on its anti-erosive/anti-abrasive efficacy—A controlled randomised in situ trial. Clin. Oral Investig. 2014, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Keegan, G.M.; Smart, J.D.; Ingram, M.J.; Barnes, L.-M.; Burnett, G.R.; Rees, G.D. Chitosan microparticles for the controlled delivery of fluoride. J. Dent. 2012, 40, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Sanko, N.; Escudero, C.; Sediqi, N.; Smistad, G.; Hiorth, M. Fluoride loaded polymeric nanoparticles for dental delivery. Eur. J. Pharm. Sci. 2017, 104, 326–334. [Google Scholar] [CrossRef]
- Wassel, M.O.; Khattab, M.A. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Giunchedi, P.; Juliano, C.; Gavini, E.; Cossu, M.; Sorrenti, M. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur. J. Pharm. Biopharm. 2002, 53, 233–239. [Google Scholar] [CrossRef]
- Peng, P.C.; Hsieh, C.M.; Chen, C.P.; Tsai, T.; Chen, C.T. Assessment of photodynamic inactivation against periodontal bacteria mediated by a chitosan hydrogel in a 3D gingival model. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan : Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska, S. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Brigham, C.J. Chitin and Chitosan: Sustainable, Medically Relevant Biomaterials. Int. J. Biotechnol. Wellness Ind. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Gross, P.; Konrad, E.; Mager, H. Hair Shampoo and Conditioning Lotion. U.S. Patent 4202.881, 13 May 1980. [Google Scholar]
- Gross, P.; Konrad, E.; Mager, H. Hair Setting Lotion Containing a Chitosan Derivative. US4134412A, 16 January 1979. [Google Scholar]
- Xiuzhen, S. Chitosan Hair Spray. CN1082883 A, 2 March 1994. [Google Scholar]
- Derks, F.J.M.; Foster, S.; Lochhead, R.; Maini, A.; Weber, D. Shampoo Preparations. US9339449 B2, 17 May 2016. [Google Scholar]
- Beumer, R.; Derks, F.; Mendrok, C. Hair Care Compositions. US20140348771 A1, 2014. [Google Scholar]
- Lang, G.; Wendel, H.; Konrad, E. Cosmetic Composition Based Upon Chitosan Derivatives, New Chitosan Derivatives as well as Processes for the Production Thereof. US4528283 A, 1985. [Google Scholar]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Maresch, G.; Lenz, H.R.; Konrad, E.; Breuer, L.; Hoch, D. Cosmetic Compositions on the Basis of Alkyl-Hydroxypropyl-Substituted Chitosan Derivatives, New Chitosan Derivatives and Processes for the Production Thereof. US4845204 A, 4 July 1989. [Google Scholar]
- Lang, G.; Wendel, H.; Konrad, E. Cosmetic Agent on the Basis of Quaternary Chitosan Derivatives, Novel Quaternary Chitosan Derivatives as well as Processes for Making Same. US4822598 A, 18 April 1989. [Google Scholar]
- Lang, G.; Wendel, H. Macromolecular, Surface-Active, Quaternary, N-Substituted Chitosan Derivatives as well as Cosmetic Composition Based on These New Chitosan Derivatives. US4976952 A, 11 December 1990. [Google Scholar]
- Gelfuso, G.M.; Gratieri, T.; Simão, P.S.; de Freitas, L.A.P.; Lopez, R.F.V. Chitosan microparticles for sustaining the topical delivery of minoxidil sulphate. J. Microencapsul. 2011, 28, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, Y.A.; El-Khordagui, L.K.; Boraei, N.A.; Darwish, I.A. Chitosan microparticles incorporating a hydrophilic sunscreen agent. Carbohydr. Polym. 2010, 81, 234–242. [Google Scholar] [CrossRef]
- Chou, K.F.; Wang, L.F. Addition of Titanium Dioxide and Sources Effects on UV Transmittance and Hydrophilicity of Chitosan Film. In Proceedings of the 5th International Conference on Biomedical Engineering and Technology; IACSIT Press: Seoul, Korea, 2015; Volume 81, pp. 20–24. [Google Scholar] [CrossRef]
- Kong, S.-Z.; Li, D.-D.; Luo, H.; Li, W.-J.; Huang, Y.-M.; Li, J.-C.; Hu, Z.; Huang, N.; Guo, M.-H.; Chen, Y.; Li, S.-D. Anti-photoaging effects of chitosan oligosaccharide in ultraviolet-irradiated hairless mouse skin. Exp. Gerontol. 2018, 103, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; Minami, S. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Yoneda, T.; Omura, Y.; Osaki, T.; Ifuku, S.; Saimoto, H.; Azuma, K.; Imagawa, T.; Tsuka, T.; Murahata, Y.; Ito, N.; Okamoto, Y.; Minami, S. Protective Effect of Chitin Urocanate Nanofibers against Ultraviolet Radiation. Mar. Drugs 2015, 13, 7076. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, G. Skin-feel agents. In Handbook of Cosmetic Science and Technology; Barel, A.O., Paye, M., Maibach, H., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Zhang, J.; Qi, Y.; Liu, Y.; Zhang, D.; Hu, X.; Li, Z. Preparation of Chitosan Derivative Cosmetic Humectant. CN1253769 A, 24 May 2000. [Google Scholar]
- Zheng, Y.; Lai, X.; Ipsen, H.; Larsen, J.N.; Løwenstein, H.; Søndergaard, I.; Jacobsen, S. Structural changes of protein antigens due to adsorption onto and release from aluminium hydroxide using FTIR-ATR. Spectroscopy 2007, 21, 211–226. [Google Scholar] [CrossRef]
- Qin, C.; Du, Y.; Xiao, L.; Liu, Y.; Yu, H. Moisture retention and antibacterial activity of modified chitosan by hydrogen peroxide. J. Appl. Polym. Sci. 2002, 86, 1724–1730. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wu, H.; Xiao, L. Relationship between molecular structure and moisture-retention ability of carboxymethyl chitin and chitosan. J. Appl. Polym. Sci. 2002, 83, 1233–1241. [Google Scholar] [CrossRef]
- Gautier, S.; Xhauflaire-Uhoda, E.; Gonry, P.; Piérard, G.E. Chitin-glucan, a natural cell scaffold for skin moisturization and rejuvenation. Int. J. Cosmet. Sci. 2008, 30, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Guo, B.; Luo, J. Quaternized carboxymethyl chitosan/organic montmorillonite nanocomposite as a novel cosmetic ingredient against skin aging. Carbohydr. Polym. 2017, 173, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrıguez, M.S.; Albertengo, L.A.; Agulló, E. Emulsification capacity of chitosan. Carbohydr. Polym. 2002, 48, 271–276. [Google Scholar] [CrossRef]
- Desbrieres, J.; Bousquet, C.; Babak, V. Surfactant-chitosan interactions and application to emulsion stabilization. Cell. Chem. Technol. 2010, 44, 395–406. [Google Scholar]
- Chen, R.H.; Heh, R.S. Skin hydration effects, physico-chemical properties and vitamin E release ratio of vital moisture creams containing water-soluble chitosans. Int. J. Cosmet. Sci. 2000, 22, 349–360. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Weckx, M.; Filippini, O.; Lough, C. Characteristic properties of N-Carboxybutyl chitosan. Carbohydr. Polym. 1989, 11, 307–320. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Chapter Two—Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 73, pp. 15–31. ISBN 1043-4526. [Google Scholar]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mengíbar, M.; Mateos-Aparicio, I.; Miralles, B.; Heras, Á. Influence of the physico-chemical characteristics of chito-oligosaccharides (COS) on antioxidant activity. Carbohydr. Polym. 2013, 97, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Jarmila, V.; Eva, V. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, P.; Müller, B.W. Chitosan emulsion formulation. EP1190702 A1, 2002. [Google Scholar]
- Champer, J.; Patel, J.; Fernando, N.; Salehi, E.; Wong, V.; Kim, J. Chitosan against cutaneous pathogens. AMB Express 2013, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, N.M.; Contri, R.V.; Paese, K.; Beck, R.C.R.; Pohlmann, A.R.; Guterres, S.S. Innovative Sunscreen Formulation Based on Benzophenone-3-Loaded Chitosan-Coated Polymeric Nanocapsules. Skin Pharmacol. Physiol. 2011, 24, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Coutinho, C.; Santos-Oliveira, R.; dos Santos, E.; Mansur, C.R. Development of a photoprotective and antioxidant nanoemulsion containing chitosan as an agent for improving skin retention. Eng. Life Sci. 2015, 15, 593–604. [Google Scholar] [CrossRef]
- Morsy, R.; Ali, S.S.; El-Shetehy, M. Development of hydroxyapatite-chitosan gel sunscreen combating clinical multidrug-resistant bacteria. J. Mol. Struct. 2017, 1143, 251–258. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, E.V.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin. Cosmetics 2014, 1. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.; Wesley, N.O. Topical Retinoids and Cosmeceuticals: Where Is the Scientific Evidence to Recommend Products to Patients? Curr Derm. Rep. 2015, 4, 56–62. [Google Scholar] [CrossRef]
- Kim, D.-G.; Jeong, Y.-I.; Choi, C.; Roh, S.-H.; Kang, S.-K.; Jang, M.-K.; Nah, J.-W. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int. J. Pharm. 2006, 319, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-E.; Park, D.-J.; Kim, B.-K. Effects of a chitosan coating on properties of retinol-encapsulated zein nanoparticles. Food Sci. Biotechnol. 2015, 24, 1725–1733. [Google Scholar] [CrossRef]
- Huang, S.-J.; Sun, S.-L.; Chiu, C.-C.; Wang, L.-F. Retinol-encapsulated water-soluble succinated chitosan nanoparticles for antioxidant applications. J. Biomater. Sci. Polym. Ed. 2013, 24, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, L.M.; Morganti, G.; Cardillo, M.; Chianese, A. Green Nanotechnology Serving the Bioeconomy: Natural Beauty Masks to Save the Environment. Cosmetics 2016, 3. [Google Scholar] [CrossRef]
- Harris, R.; Lecumberri, E.; Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2011, 84, 803–806. [Google Scholar] [CrossRef]
- Anchisi, C.; Meloni, M.C.; Maccioni, A.M. Chitosan beads loaded with essential oils in cosmetic formulations. J. Cosmet. Sci. 2006, 57, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. J. Food Hydrocoll 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Lee, S.-M.; Liu, K.-H.; Liu, Y.-Y.; Chang, Y.-P.; Lin, C.-C.; Chen, Y.-S. Chitosonic(®) Acid as a Novel Cosmetic Ingredient: Evaluation of its Antimicrobial, Antioxidant and Hydration Activities. Materials (Basel) 2013, 6, 1391–1402. [Google Scholar] [CrossRef] [PubMed]




| (i) A Cosmeceutical is a scientifically designed product intended for external application to the human body |
| (ii) A Cosmeceutical produces a useful and desired result |
| (iii) A Cosmeceutical has desirable aesthetic properties |
| (iv) A Cosmeceutical meets rigid chemical, physical and medical standards |
| Ingredient | Source | Activity/Use |
|---|---|---|
| Alginate | Seaweed (brown algae) | Texture and emulsion stabilizer Vehicle for controlled delivery Thickening agent |
| Fucoidans | Wound-healing | |
| Phlorotannin | Sunscreen and antioxidant activities | |
| Fucoxanthin | UV protective and antioxidant activities | |
| Carrageenan | Seaweed (red algae) | Viscosity altering Thickening agent |
| MAAs | Antioxidant | |
| Ulvans | Seaweed (green algae) | Antioxidant |
| Glycogen | Mussel | UVB protection Moisturizing |
| Aluminium silicate | Sea mud | Absorbent |
| Squalene | Shark | Skin lubrication |
| Chitin | Crustaceans shells | Vehicle for controlled delivery Antiaging Skin protecting |
| Chitosan | Crustaceans shells | Vehicle for controlled delivery Antimicrobial Antioxidant Emulsifying Skin protecting |
| Vehicle | Characteristics |
|---|---|
| Mouth rinses | Simplest vehicle formulation Compatible with most antimicrobial agents |
| Sprays | Relatively small doses to achieve efficacy Good compliance Easy usage |
| Dentifrices | Complex formulation Possible interaction among components Tooth brushing with a dentifrice is a well-adopted habit |
| Gels | Thickened aqueous system Non-abrasive No foaming agents Compatible with relevant antimicrobials Specific devices are needed for applications |
| Chewing gum/lozenges | Larger contact time Stimulated salivary secretion Useful for patients with low tooth-brushing compliance |
| Sustained-release formulations/devices | Long-term effect The efficacy is independent of patient compliance |
| Low stickiness |
| Lack of powdering or flaking |
| Preferably being clear |
| Preferably transparent |
| Preferably glossy |
| Good film formation |
| Good holding power |
| High level of style retention |
| Prolonged curl retention |
| Improved combability |
| Easily removed upon washing the hair |
| Bacterial Strain | Chitosan Properties | MIC mg/mL | |
|---|---|---|---|
| Mw, kDa | DA | ||
| S. mutants | 1400 | 0.2 | 0.08 |
| 1080 | 0.14 | 2.5 | |
| 624 | <0.25 | 3 | |
| 107 | 0.15–0.25 | 5 | |
| P. intermedia | 1080 | 0.14 | 2.5 |
| 624 | <0.25 | 1 | |
| 107 | 0.15–0.25 | 3 | |
| P. buccae | 624 | <0.25 | 3 |
| 107 | 0.15–0.25 | 1 | |
| T. forythensis | 624 | <0.25 | 1 |
| 107 | 0.15–0.25 | 3 | |
| A. actinomycetemcomitans | 1080 | 0.14 | 2.5 |
| 624 | <0.25 | 5 | |
| 107 | 0.15–0.25 | 3 | |
| P. gingivalis | 1080 | 0.14 | 0.5 |
| 624 | <0.25 | 1 | |
| 272 | 0.05 | 3.8 | |
| 272 | 0.16 | 3.8 | |
| 272 | 0.27 | 3.6 | |
| 107 | 0.15–0.25 | 1 | |
| Polymer Derivative | Bacterial Strain | Effect |
|---|---|---|
| Ethylenglycol chitin | S. mutants S. sanguis S mitis | Reduce bacterial adsorption on S-HA in vitro Dose dependent effect Better activity on S. mutants |
| Carboxymethyl chitin | S. mutants S. sanguis S mitis | Reduce bacterial adsorption on S-HA in vitro Dose dependent effect Better activity on S. mutants |
| N-Carboxymethyl chitosan | S mutants | Prevent bacterial adsorption to HA in vitro |
| S. sanguis, S. gordonii, S. constellatus, S. anginosus, S. intermedius, S. oralis, S. salivarius, S. vestibularis | Adsorption reduction on HA and S-HA (60%–98%) in vitro | |
| Imidazolyl chitosan | S mutants | Prevent bacterial adsorption to HA in vitro |
| S. sanguis, S. gordonii, S. constellatus, S. anginosus, S. intermedius, S. oralis, S. salivarius, S. vestibularis | No effect on bacterial adhesion to HA or S-HA in vitro | |
| Sulphated chitosan | S. muntants S. sanguis S. mitis | Reduce bacterial adsorption on S-HA in vitroDose dependent effect. Better activity on S. mutants |
| Phosphorylated chitosan | S. muntants S. sanguis S. mitis | Reduce bacterial adsorption on S-HA in vitro. Dose dependent effect. Better activity on S. mutants Plaque reduction Slight plaque buffering capacity |
| N-1hydroxy 3 trimethyl ammonium chitosan HCl | P. gingivalis P. intermedia A. actinomycetemcomitans S. mutans | Antibacterial activity in vitro MIC: 0.5–1 mg/mL |
| Glucosamine Maillard chitosan derivative | S. mutants L. brevis | CBM S. mutants 0.4 mg/mL CBM L. brevis 0.5 mg/mL No cytotoxicity in vivo |
| Water-soluble reduced chitosan | S. mutans S sanguinis | MIC S mutans 1.25 mg/mL MIC S sanguinis 10 mg/mL Reduction plaque index Reduction vital fluorescence |
| Requisite | Chitosan | Water Soluble Derivatives |
|---|---|---|
| Heat stability | Up to 170 °C | To be checked |
| Very good solubility | Only acidic media, depends on DA and Mw | Yes |
| Compatibility with cosmetic bases | Yes | To be checked |
| pH stability in the range of 4 to 9 | Yes | To be checked |
| Processability into a variety of products | Yes | Yes |
| Compatibility with other ingredients and with the packaging materials | Yes | Yes |
| Free of colour | White to yellowish | To be checked |
| Neutral or pleasant odour | Yes | Yes |
| Low volatility | Non-volatile | Non-volatile |
| Hair Conditioning | Film Forming |
|---|---|
| Butoxy CH Carboxybutyl CH Carboxymethyl CH Succinamide CHT Propylsulfonate Hydrolyzed CHT CHT Hydroxypropyltrimonium Chloride CHT Isostearamide Hydroxypropyltrimonium Chloride CHT Lauramide Succinamide CHT Lauroyl Glycinate CHT Rice Branamide Hydroxypropyltrimonium Chloride Sodium Carboxymethyl CHT Lauramide Sodium CHT Caprylamide Hydroxypropylsulfonate Sodium CHT Cocamide Hydroxypropylsulfate Sodium CHT Cocamide Hydroxypropylsulfonate Sodium CHT Isostearamide Hydroxypropylsulfonate Sodium CHT Lauramide Hydroxypropylsulfate Sodium CHT Lauramide Hydroxypropylsulfonate Sodium CHT Rice Branamide Hydroxypropylsulfonate Sodium CHT Stearamide Hydroxypropylsulfonate Carboxymethyl CH | Butoxy CH Calcium CH Carboxybutyl CH Carboxymethyl Caprooyl CHT Carboxymethyl CHT Carboxymethyl CHT Succinamide CHT Adipate CHT Ascorbate CHT Formate CHT Glycolate CHT Lactate CHT PCA Palmitamide Succinamide CHT Propylsulfonate CHT Salicylate CHT Succinamide Hydrolyzed CH Hydroxyethyl CHTT Hydroxypropyl CHT Polyquaternium-29 |
| Skin Conditioning | Emollient |
|---|---|
| Calcium CHT Carboxybutyl CHT Carboxymethyl Caprooyl CHT Carboxymethyl CHT Succinamide CHT Ascorbate CHT Caprylamide Hydroxypropyl trimonium Chloride Hydrolyzed CHT Hydrolyzed CHT Ferulyl Linoleate Myristoyl/PCA CH Polyquaternium-29 Sodium Carboxymethyl CHT Lauramide Sodium CHT Cocamide Hydroxypropyl sulfate Sodium CH Cocamide Hydroxypropyl sulfonate Sodium CHT Isostearamide Hydroxypropyl sulfonate Sodium CHT Lauramide Hydroxypropyl sulfate Sodium CHT Lauramide Hydroxypropyl sulfonate Sodium CHT Rice Branamide Hydroxypropyl sulfonate Sodium CHT Stearamide Hydroxypropyl sulfonate Sodium Carboxymethyl CH CHT Glycolate CHT Isostearamide Hydroxypropyl trimonium Chloride CHT Lauramide Hydroxypropyltrimonium Chloride CHT PCA Palmitamide Succinamide CHT Propylsulfonate CHT Rice Branamide Hydroxypropyl trimonium Chloride CHT Salicylate Carboxymethyl CH CH glycolate CH sulfate Hydrolyzed CH Mystoil PCA CH | CHT Rice Branamide Hydroxypropyl trimonium Chloride Sodium CHT Caprylamide Hydroxypropyl sulfonate Sodium CHT Cocamide Hydroxypropyl sulfate Sodium CHT Cocamide Hydroxypropyl sulfonate Sodium CHT Isostearamide Hydroxypropyl sulfonate Sodium CHT Rice Branamide Hydroxypropyl sulfonate Sodium CHT Stearamide Hydroxypropyl sulfonate CHT Caprylamide Hydroxypropyl trimonium Chloride |
| Humectant | Moisturizing |
|---|---|
| Carboxymethyl Caprooyl CHT | CHT Rice Branamide Hydroxypropyltrimonium Cl |
| Carboxymethyl CHT Myristamide | Sodium CHT Cocamide Hydroxypropylsulfate |
| Carboxymethyl CHT Succinamide | Sodium CH Cocamide Hydroxypropylsulfonate |
| CHT Hydroxypropyltrimonium Cl | Sodium CHT Isostearamide Hydroxypropylsulfonate |
| CHT Lauroyl Glycinate | Sodium CHT Rice Branamide Hydroxypropylsulfonate |
| CHT PCA Palmitamide Succinamide | Sodium CHT Stearamide Hydroxypropylsulfonate |
| CH sulfate | CHT Caprylamide Hydroxypropyltrimonium Cl |
| Sodium Carboxymethyl CH | Carboxybutyl CHT |
| Carboxymethyl CHT Succinamide | |
| CHT Propylsulfonate | |
| Hydrolyzed CHT | |
| CHT Isostearamide Hydroxypropyltrimonium Cl | |
| Sodium Carboxymethyl CHT Lauramide | |
| Sodium CHT Lauramide Hydroxypropylsulfate | |
| Sodium CHT Lauramide Hydroxypropylsulfonate | |
| Calcium CHT | |
| Carboxymethyl Caprooyl CHT | |
| CHT Ascorbate | |
| CHT Glycolate | |
| CHT PCA Palmitamide Succinamide | |
| CHT Salicylate | |
| Polyquaternium-29 | |
| CHT Lauramide Hydroxypropyltrimonium Cl | |
| CHT PCA Palmitamide Succinamide | |
| Hydrolyzed CHT Ferulyl Linoleate | |
| Myristoyl/PCA CH |
| Surfactant | Emulsifier | Viscosifier |
|---|---|---|
| Carboxymethyl Caprooyl CHT CHT Argininamide CHT Lauramide Hydroxypropyl trimonium Cl CHT Stearamide Hydroxypropyl trimonium Cl Sodium Carboxymethyl CHT Lauramide Sodium CHT Caprylamide Hydroxypropyl sulfonate Sodium CHT Cocamide Hydroxypropyl sulphate Sodium CHT Cocamide Hydroxypropyl sulfonate Sodium CHT Lauramide Hydroxypropyl sulphate Sodium CHT Rice Branamide Hydroxypropyl sulfonate Sodium CHT Stearamide Hydroxypropyl sulfonate | CHT Isostearamide Hydroxypropyl trimonium Cl CHT Lauramide Hydroxypropyl trimonium Cl CHT PCA Palmitamide Succinamide CHT Rice Branamide Hydroxypropyl trimonium Cl CHT Stearamide Hydroxypropyl trimonium Cl Sodium Carboxymethyl CHT Lauramide Sodium CHT Caprylamide Hydroxypropyl sulfonate Sodium CHT Cocamide Hydroxypropyl sulphate Sodium CHT Cocamide Hydroxypropyl sulfonate Sodium CHT Lauramide Hydroxypropyl sulphate Sodium CHT Lauramide Hydroxypropyl sulfonate Sodium CHT Rice Branamide Hydroxypropyl sulfonate | Carboxymethyl CH Butoxy CHT Carboxybutyl CHT Carboxymethyl CHT Hydroxyethyl CHT |
| Antioxidant | Antimicrobial |
|---|---|
| CHT Ascorbate CHT Glycolate CHT Salicylate Hydrolysed CHT Ferulyl Linoleate | Hydrolysed CHT Ferulyl Linoleate CHT Benzamide |
| Field | Property | Effect of Physico-Chemical Properties |
|---|---|---|
| Oral healthcare | ability to inhibit pH fall in dental plaque | In vitro: No effect of Mw or DA in buffering capacity. In vivo: best sample 3000 Da, DA: 0.05 |
| Oral healthcare | Antimicrobial Activity | Depends on Mw, DA and bacterial strain. Very active against S. mutants and other oral streptococci. |
| Oral healthcare | Bacterial adsorption inhibition | Dual-species inhibition (High Mw :624 kDa, DA < 0.25). Inhibition S. sobrinus High Mw, optimum DA 0.40–0.50 |
| Product conservation | Antioxidant | Best activity COS. Chitosanase- COS are preferred over lysozyme-COS |
| Product conservation | Antimicrobial Activity | Depends on Mw, DA, bacterial strain |
| Skin care | Antioxidant | Best activity COS Chitosanase-COS are preferred over lysozyme-COS |
| Skin care (Acne treatment) | Antimicrobial activity | High Mw |
| Skin/hair care | Humectant | Low DA |
| Skin/hair care | Moisturizing agent | High Mw Carboxylmethyl derivatives |
| Skin care | Sunscreen | Possible effect of the chitosan source |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.l.L.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. https://doi.org/10.3390/polym10020213
Aranaz I, Acosta N, Civera C, Elorza B, Mingo J, Castro C, Gandía MDlL, Heras Caballero A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers. 2018; 10(2):213. https://doi.org/10.3390/polym10020213
Chicago/Turabian StyleAranaz, Inmaculada, Niuris Acosta, Concepción Civera, Begoña Elorza, Javier Mingo, Carolina Castro, María De los Llanos Gandía, and Angeles Heras Caballero. 2018. "Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives" Polymers 10, no. 2: 213. https://doi.org/10.3390/polym10020213
APA StyleAranaz, I., Acosta, N., Civera, C., Elorza, B., Mingo, J., Castro, C., Gandía, M. D. l. L., & Heras Caballero, A. (2018). Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers, 10(2), 213. https://doi.org/10.3390/polym10020213