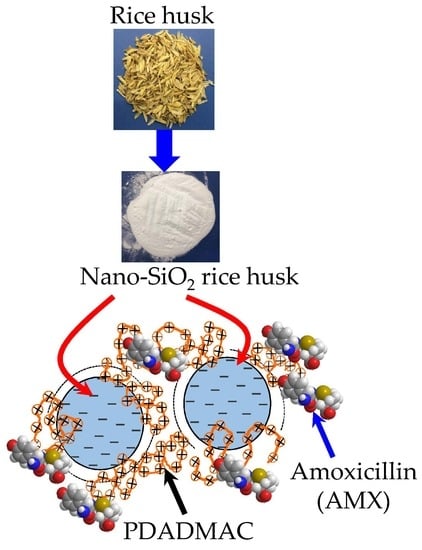
Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Nanosilica from Rice Husk
2.3. Characterization Methods
2.4. Adsorption Studies
3. Results and Discussion
3.1. Characterizations of Nanosilica from Rice Husk
3.2. Adsorption of PDADMAC onto Nanosilica
3.2.1. Effect of pH
3.2.2. Effect of Ionic Strength
3.2.3. Adsorption Isotherms
3.2.4. Adsorption Mechanisms
3.3. Adsorptive Removal of Amoxicillin Using PDADMAC-Modified Nanosilica
3.3.1. Influence of Contact Time
3.3.2. Influence of pH
3.3.3. Influence of Adsorbent Dosage
3.4. Adsorptive Removal of AMX onto Silica without and with Surface Modification with PDADMAC
3.5. Comparison of the Effectiveness of PMS and Other Adsorbents
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ayodele, O.B. Effect of phosphoric acid treatment on kaolinite supported ferrioxalate catalyst for the degradation of amoxicillin in batch photo-Fenton process. Appl. Clay Sci. 2013, 72, 74–83. [Google Scholar] [CrossRef]
- Fakhri, A.; Adami, S. Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J. Taiwan Inst. Chem. Eng. 2014, 45, 1001–1006. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem. Eng. J. 2015, 260, 550–556. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Akmehmet Balcıoğlu, I.; Ötker, M. Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes. Chemosphere 2003, 50, 85–95. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Guillard, C.; Pichat, P. Heterogeneous photocatalysis: An emerging technology for water treatment. Catal. Today 1993, 17, 7–20. [Google Scholar] [CrossRef]
- Santiago-Morales, J.; Gómez, M.J.; Herrera-López, S.; Fernández-Alba, A.R.; García-Calvo, E.; Rosal, R. Energy efficiency for the removal of non-polar pollutants during ultraviolet irradiation, visible light photocatalysis and ozonation of a wastewater effluent. Water Res. 2013, 47, 5546–5556. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.M.; Manaia, C.M.; Mendes, A.; Nunes, O.C. Photoinactivation of various antibiotic resistant strains of Escherichia coli using a paint coat. J. Photochem. Photobiol. Chem. 2013, 251, 148–153. [Google Scholar] [CrossRef]
- Dutta, M.; Dutta, N.N.; Bhattacharya, K.G. Aqueous phase adsorption of certain beta-lactam antibiotics onto polymeric resins and activated carbon. Sep. Purif. Technol. 1999, 16, 213–224. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of Polyanion onto Large Alpha Alumina Beads with Variably Charged Surface. Adv. Phys. Chem. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of anionic surfactant sodium dodecyl sulfate onto alpha alumina with small surface area. Colloid Polym. Sci. 2015, 293, 217–227. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of anionic azo dye onto large α-alumina beads. Colloid Polym. Sci. 2015, 293, 1877–1886. [Google Scholar] [CrossRef]
- Zha, S.X.; Zhou, Y.; Jin, X.; Chen, Z. The removal of amoxicillin from wastewater using organobentonite. J. Environ. Manag. 2013, 129, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Ribau Teixeira, M.; Camacho, F.P.; Sousa, V.S.; Bergamasco, R. Green technologies for cyanobacteria and natural organic matter water treatment using natural based products. J. Clean. Prod. 2017, 162, 484–490. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Figueroa, R.A.; MacKay, A.A. Sorption of Oxytetracycline to Iron Oxides and Iron Oxide-Rich Soils. Environ. Sci. Technol. 2005, 39, 6664–6671. [Google Scholar] [CrossRef] [PubMed]
- Mon, J.; Flury, M.; Harsh, J.B. Sorption of four triarylmethane dyes in a sandy soil determined by batch and column experiments. Geoderma 2006, 133, 217–224. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H. Adsorption behavior of antibiotic in soil environment: A critical review. Front. Environ. Sci. Eng. 2015, 9, 565–574. [Google Scholar] [CrossRef]
- Pham, T.D.; Do, T.T.; Ha, V.L.; Doan, T.H.Y.; Nguyen, T.A.H.; Mai, T.D.; Kobayashi, M.; Adachi, Y. Adsorptive removal of ammonium ion from aqueous solution using surfactant-modified alumina. Environ. Chem. 2017, 14, 327–337. [Google Scholar] [CrossRef]
- Adak, A.; Pal, A.; Bandyopadhyay, M. Spectrophotometric determination of anionic surfactants in wastewater using acridine orange. Ind. J. Chem. Technol. 2005, 12, 145. [Google Scholar]
- Aloulou, F.; Boufi, S.; Beneventi, D. Adsorption of organic compounds onto polyelectrolyte immobilized-surfactant aggregates on cellulosic fibers. J. Colloid Interface Sci. 2004, 280, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Mishael, Y.G.; Dubin, P.L. Uptake of Organic Pollutants by Silica−Polycation-Immobilized Micelles for Groundwater Remediation. Environ. Sci. Technol. 2005, 39, 8475–8480. [Google Scholar] [CrossRef] [PubMed]
- Mishael, Y.G.; Dubin, P.L.; De Vries, R.; Kayitmazer, A.B. Effect of Pore Size on Adsorption of a Polyelectrolyte to Porous Glass. Langmuir 2007, 23, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Zembala, M.; Michna, A. Polyelectrolyte adsorption layers studied by streaming potential and particle deposition. J. Colloid Interface Sci. 2006, 303, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Blokhus, A.M.; Djurhuus, K. Adsorption of poly(styrene sulfonate) of different molecular weights on α-alumina: Effect of added sodium dodecyl sulfate. J. Colloid Interface Sci. 2006, 296, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, N.G.; Stuart, M.A.C.; Fleer, G.J. Polyelectrolyte Adsorption on Oxides: I. Kinetics and Adsorbed Amounts. J. Colloid Interface Sci 1996, 182, 133–145. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Vu, C.M.; Choi, H.J. Enhanced fracture toughness and mechanical properties of epoxy resin with rice husk-based nano-silica. Polym. Sci. Ser. A 2017, 59, 437–444. [Google Scholar] [CrossRef]
- Wang, Y.; Banziger, J.; Dubin, P.L.; Filippelli, G.; Nuraje, N. Adsorptive Partitioning of an Organic Compound onto Polyelectrolyte-Immobilized Micelles on Porous Glass and Sand. Environ. Sci. Technol. 2001, 35, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- Zadaka, D.; Mishael, Y.G.; Polubesova, T.; Serban, C.; Nir, S. Modified silicates and porous glass as adsorbents for removal of organic pollutants from water and comparison with activated carbons. Appl. Clay Sci. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Guzman, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyl-dimethylammonium chloride) and poly(4-styrene sulfonate of sodium). Soft Matter 2009, 5, 2130–2142. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in Lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Zhu, B.-Y.; Gu, T. Surfactant adsorption at solid-liquid interfaces. Adv. Colloid Interface Sci. 1991, 37, 1–32. [Google Scholar] [CrossRef]
- Hoffmann, I.; Oppel, C.; Gernert, U.; Barreleiro, P.; Von Rybinski, W.; Gradzielski, M. Adsorption Isotherms of Cellulose-Based Polymers onto Cotton Fibers Determined by Means of a Direct Method of Fluorescence Spectroscopy. Langmuir 2012, 28, 7695–7703. [Google Scholar] [CrossRef] [PubMed]
- Ndong, R.; Russel, W. Linear viscoelasticity of ZrO2 nanoparticle dispersions with associative polymers. Rheol. Acta 2012, 51, 771–782. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.A.; Ortega, F.; Rubio, R.G. Growth of Polyelectrolyte Layers Formed by Poly(4-styrenesulfonate sodium salt) and Two Different Polycations: New Insights from Study of Adsorption Kinetics. J. Phys. Chem. C 2012, 116, 15474–15483. [Google Scholar] [CrossRef]
- Kobayashi, M.; Skarba, M.; Galletto, P.; Cakara, D.; Borkovec, M. Effects of heat treatment on the aggregation and charging of Stöber-type silica. J. Colloid Interface Sci. 2005, 292, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef] [PubMed]
- Kannan, C.; Sundaram, T.; Palvannan, T. Environmentally stable adsorbent of tetrahedral silica and non-tetrahedral alumina for removal and recovery of malachite green dye from aqueous solution. J. Hazard. Mater. 2008, 157, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Čakara, D.; Kobayashi, M.; Skarba, M.; Borkovec, M. Protonation of silica particles in the presence of a strong cationic polyelectrolyte. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 20–25. [Google Scholar] [CrossRef]
- Matsumoto, T.; Adachi, Y. Effect of Ionic Strength on the Initial Dynamics of Flocculation of Polystyrene Latex with Polyelectrolyte. J. Colloid Interface Sci. 1998, 204, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, R.; Thompson, L.; Bos, M.; De Groot, P. Adsorption and Electrokinetic Properties of Polyethylenimine on Silica Surfaces. Langmuir 2002, 18, 6164–6169. [Google Scholar] [CrossRef]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-Induced Changes in the Fabrication of Multilayers of Poly(acrylic acid) and Chitosan: Fabrication, Properties, and Tests as a Drug Storage and Delivery System. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. Electrophoretic mobility of latex spheres in the presence of divalent ions: experiments and modeling. Colloid Polym. Sci. 2008, 286, 935–940. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, A.; Kobayashi, M. Quantitative evaluation of shift of slipping plane and counterion binding to lysozyme by electrophoresis method. Colloid Polym. Sci. 2016, 294, 1019–1026. [Google Scholar] [CrossRef]
- Huang, Y.; Yamaguchi, A.; Pham, T.D.; Kobayashi, M. Charging and aggregation behavior of silica particles in the presence of lysozymes. Colloid Polym. Sci. 2018, 296, 145–155. [Google Scholar] [CrossRef]
- Kobayashi, M.; Juillerat, F.; Galletto, P.; Bowen, P.; Borkovec, M. Aggregation and Charging of Colloidal Silica Particles: Effect of Particle Size. Langmuir 2005, 21, 5761–5769. [Google Scholar] [CrossRef] [PubMed]
- Kochameshki, M.G.; Marjani, A.; Mahmoudian, M.; Farhadi, K. Grafting of diallyldimethylammonium chloride on graphene oxide by RAFT polymerization for modification of nanocomposite polysulfone membranes using in water treatment. Chem. Eng. J. 2017, 309, 206–221. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Adsorption of ammonia on graphite oxide/aluminium polycation and graphite oxide/zirconium–aluminium polyoxycation composites. J. Colloid Interface Sci. 2008, 324, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, F.; Jalali, M. Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J. Environ. Chem. Eng. 2016, 4, 1664–1673. [Google Scholar] [CrossRef]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Chiew, C.S.C.; Yeoh, H.K.; Pasbakhsh, P.; Poh, P.E.; Tey, B.T.; Chan, E.S. Stability and reusability of alginate-based adsorbents for repetitive lead (II) removal. Polym. Degrad. Stab. 2016, 123, 146–154. [Google Scholar] [CrossRef]
- Saiz, J.; Bringas, E.; Ortiz, I. New Functionalized Magnetic Materials for As5+ Removal: Adsorbent Regeneration and Reuse. Ind. Eng. Chem. Res. 2014, 53, 18928–18934. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Sadegh, N. Effective removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Adriano, W.S.; Veredas, V.; Santana, C.C.; Gonçalves, L.R.B. Adsorption of amoxicillin on chitosan beads: Kinetics, equilibrium and validation of finite bath models. Biochem. Eng. J. 2005, 27, 132–137. [Google Scholar] [CrossRef]
- Jin, X.; Zha, S.; Li, S.; Chen, Z. Simultaneous removal of mixed contaminants by organoclays—Amoxicillin and Cu(II) from aqueous solution. Appl. Clay Sci. 2014, 102, 196–201. [Google Scholar] [CrossRef]
















| CKCl (mM) | Γ∞ (mg/g) | k1 (g/mg) | k2 (g/mg) n−1 | n |
|---|---|---|---|---|
| 10 | 50 | 2.1 | 0.1 | 3.6 |
| 100 | 64 | 50 | 0.1 | 3.5 |
| Adsorbent | Adsorption Capacity (mg/g) | Removal Efficiency (%) | References |
|---|---|---|---|
| Activated carbon | 7.367 | 50.26 | [60] |
| Chitosan beads | 8.71 | NF | [61] |
| Organobentonite | 26.18 | 61.5 | [13] |
| Bentonite | 18.75 | 88.01 | [31] |
| Organoclays | 24.29 | 34.80 | [62] |
| PMS | 7.50 | 92.30 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.D.; Bui, T.T.; Nguyen, V.T.; Bui, T.K.V.; Tran, T.T.; Phan, Q.C.; Pham, T.D.; Hoang, T.H. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers 2018, 10, 220. https://doi.org/10.3390/polym10020220
Pham TD, Bui TT, Nguyen VT, Bui TKV, Tran TT, Phan QC, Pham TD, Hoang TH. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers. 2018; 10(2):220. https://doi.org/10.3390/polym10020220
Chicago/Turabian StylePham, Tien Duc, Thu Thuy Bui, Van Thanh Nguyen, Thi Kieu Van Bui, Thi Thuy Tran, Quynh Chi Phan, Tien Dat Pham, and Thu Ha Hoang. 2018. "Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal" Polymers 10, no. 2: 220. https://doi.org/10.3390/polym10020220
APA StylePham, T. D., Bui, T. T., Nguyen, V. T., Bui, T. K. V., Tran, T. T., Phan, Q. C., Pham, T. D., & Hoang, T. H. (2018). Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers, 10(2), 220. https://doi.org/10.3390/polym10020220