1. Introduction
Processing of chitosan and chitosan blended with different polymers using electrospinning is often proposed in the literature to produce new biomaterials, especially developed for biomedical applications [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. The advantage of chitosan is that it is obtained from a natural polymer, chitin, after controlled deacetylation. Chitosan is soluble in aqueous medium in acidic conditions due to –NH
2 protonation as soon as its degree of acetylation is lower than 0.5 [
11]. Then, processing of chitosan is relatively easy, and it can be used under fiber, nanofiber, film, capsule, bead, sponge, gel, powder, tablet based on its insolubility in neutral medium.
Additionally, chitosan is an interesting biodegradable and biocompatible polymer with antibacterial and antifungal properties often described in the literature [
12]. In addition, chitosan is stabilized by H–bond network in the solid state providing good mechanical properties under film or fiber materials. The main applications proposed for chitosan in the biomedical domain are: drug delivery, gene delivery vehicle, encapsulation of sensitive drugs, medical textiles, guided bone regeneration, scaffold for nerve tissue regeneration, and wound healing.
In a previous work, the conditions for electrospinning of pure chitosan nanofibers were optimized considering conditions published in the literature [
13]. Electrospinning of chitosan has drawn a lot of attention in several scientific studies and a wide range of methods have been used to produce chitosan-based nanofibrous materials in the presence of poly (ethylene oxide) (PEO) as mentioned previously [
13,
14,
15,
16,
17,
18,
19]. The optimum condition for a good spinnability and solubility of chitosan was obtained by using 0.5 M acetic acid as solvent and blending with PEO either in solution or in powder. Compatibility of chitosan and PEO was also shown and it was demonstrated that interaction between PEO and chitosan favored the processing. Nanofibers were obtained in the presence of 20 up to 40% (
w/
w) PEO yield solutions. In addition, it was confirmed that a chitosan sample with moderate molar mass (
MW ~ 100,000) is more convenient to control the viscosity of the spinnable solution. In these conditions, nanofibers made of blend PEO/chitosan have average diameters under dried state between 100 and 150 nm [
13].
The advantage of electrospinning is that materials are easily produced with high porosity and large surface area which favors the cell development [
20,
21,
22]. Due to its good adhesive characteristic, chitosan is usually blended with other polymers [
21]. One reference introduces dibasic sodium phosphate as ionic cross-linker [
23]. In these conditions, based on its mechanical characteristics, the chitosan mats are adapted for soft tissue regeneration [
23]. The chitosan characteristics are recognized to be used as potential wound dressing for skin lesions due to mechanical properties in the range of that of normal skin [
24]. It is very recently that the mechanical properties of these electrospun chitosan-based nanofibers were discussed in literature. It was separately demonstrated that lower degree of acetylation increases the strength of the fibers and decreases the elongation at break for a chitosan/polyvynylalcohol (PVA) (ratio 1/1) electrospun membrane. These authors found a tensile strength at break between 3.5 and 5.2 MPa with 8% and 4% elongation at break when the degrees of acetylation are 16 and 8% respectively [
25]. The content of chitosan in the blend chitosan/PVA has also shown to increase the Young modulus and stress at break and to decrease the elongation at break [
20]. The values given at 30% of chitosan in the material gives respectively
E = 201.7 Pa,
4.15 MPa and
3.96% [
26]. For a chitosan/gelatin system (weight ratio 1/1), these parameters are respectively 48 MPa, 0.478 MPa and 1.3% [
27]. Up to now, to our knowledge, no paper concerning the mechanical properties of pure chitosan electrospun nanofibers in dry and wet states are available in the literature. The only published results on wet state concern poly (
l-lactide) nanofibers immersed in chitosan and treated with polydopamine at pH = 8.5. Chitosan decreases the stress at break and the elongation. At dried state, in presence of chitosan, the stress at break is around 2 MPa and the elongation is between 40% and 80%. The characteristics of the same samples in the wet state decreases for
in the range of 0.6–1.0 MPa and increases slowly for
up to 50% to 120% [
21]. Nevertheless, the conditions for determination of these important parameters are usually not described.
In the present work, the electrospinning of pure chitosan-based nanofibers was extended to prepare chitosan nanofiber mats and determine their mechanical properties. As reference, films were prepared from the same solutions as used for electrospinning by casting allowing us to compare their physical properties with that of the nanofibers. It will be interesting to test the main differences between unorganized casted and fibrous materials.
For fibers and films production, the two different techniques previously proposed were used: (i) solutions of PEO and solutions of chitosan were mixed and casted in a mold or electrospun; (ii) powdered PEO was added into chitosan solutions [
13]. Acetic acid at 0.5 M concentration was adopted as solvent. The change of PEO and chitosan weight ratio and PEO molar mass in the chitosan blends were investigated and related with the electrospun nanofibers and films physical properties under dried and wet conditions.
2. Materials and Methods
2.1. Materials
Chitosan (CS) sample from Northern cold-water shrimp, Pandalus borealis is a gift from Primex Cy (Batch TM4778, code 42010, Siglufjordur, Iceland). Its molecular weight (MW) is around 200 kg/mol and its degree of acetylation determined using 1H NMR is degree of acetylation (DA) = 0.05. The viscosity of 1% solution is 86 mPa in 1% acetic acid at 25 °C. Poly (ethylene oxide) (PEO) with different molecular weight MW (5 × 103 and 1 × 103 kg/mol), acetic acid (≥99.7%), ethanol and K2CO3 were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). Deionized water was used as solvent to make up the solutions. All reagents and polymers were used as received without further purification.
2.2. Sample Preparation
Chitosan (CS) solutions were prepared separately at 5% (w/v) in 0.5 M acetic acid. These solutions were prepared at room temperature with slow stirring for 4 days to obtain homogeneous solutions. In the same manner, poly (ethylene oxide) with different MW (5 × 103 and 1 × 103 kg/mol) were solubilized at 5% and 3% w/v in 0.5 M acetic acid on rotating stirrer. Chitosan (CS) solutions were mixed with the solution of PEO at chitosan/PEO weight ratio (in %) of 95/5, 90/10, 80/20, 70/30 and 60/40. Similarly, the same concentration of CS was blended with powdered PEO. The weight ratios were expressed as weight of chitosan or PEO divided by the total polymer weight for each system tested. This addition of powder (PEO) in the solution of chitosan was selected to avoid the dilution of the final chitosan concentration in the polymer blend solution when mixing the two polymers solutions. Viscosities and ionic conductivities of the blended CS/PEO solutions were measured at room temperature.
2.3. Chitosan Stabilization
Weighted initial nanofiber mats or films cut in pieces were immersed in alkaline ethanol/water (70/30) mixture dissolving K2CO3 at pH = 12 to neutralize the chitosan. Further, nanofibers membranes or films were washed for 3 days four times in a day with deionized water until neutral pH to obtain removal of the salt formed from chitosan solutions (potassium acetate), K2CO3 excess and PEO. At last, the membranes were dried at room temperature for further determination of the swelling capacity or rehydration after a first drying of the materials.
2.4. Casting of Chitosan/PEO Films
A certain amount of each of the CS/PEO mixtures showing good spinnability was placed in a Teflon mold of known volume to obtain a uniform polymer film. The probes were stored at room temperature for 3 days until complete evaporation of the solvent.
Different samples of a regular shape were taken from the films obtained for future measurements of their mechanical properties and degree of swelling.
2.5. Electrospinning
The prepared solutions were placed in a 5 mL plastic syringe fitted with a 21-gauge stainless steel needle. The syringe pump delivers solutions at specified flow rate vertically (model: KDS Legato 200, KD Scientific, Holliston, MA, USA), and electrospinning is realized with an applied voltage around 20 kV between the electrodes using a homemade dual high voltage power supplier (±30 kV, iseq GmbH, Radeberg, Germany). Then, the nanofibers were recovered on a microstructured collector with a regular pattern supporting an aluminum film used as collector and kept from 10 to 17 cm from the tip of the needle. The flow rates vary from 0.7 to 1.5 mL/h. The experiments were carried out at room temperature in closed Plexiglas® box with relative humidity ranging between 40% and 60%. The produced nanofibers matrices were left in ambient conditions to evaporate excess of acetic acid and water prior to further analyses.
2.6. Characterization of Nanofibers
2.6.1. Morphology of the Nanofibers Membranes
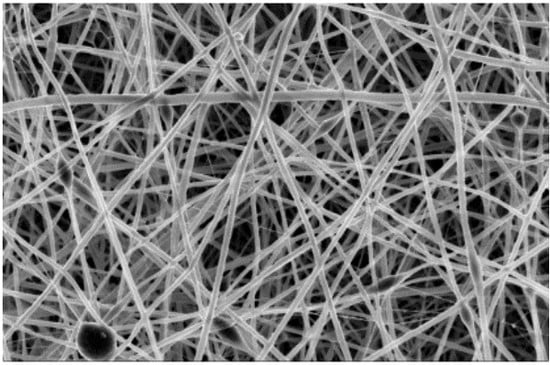
The Scanning electron microscopy (SEM) analyses of the samples were performed at CMTC-INP, Grenoble, France. The morphology of electrospun nanofiber membranes including the washed samples were observed with a scanning electron microscope (ultra 55 SEM FEG, Zeiss, Jena, Germany) operated at 3 kV. The nanofibers samples were coated with 10 nm carbon layer prior to SEM imaging. The average fiber diameter (AFD) was calculated by randomly selected diameter of 500 nanofibers from each sample.
2.6.2. Determination of Swelling Capacity
The swelling of the nanofibrous membranes or films were examined in terms of water loss between swollen state in water at neutral pH and final dried weight at room temperature. The wet swollen samples were weighed after blotting with tissue paper to remove excess surface water (
Ww). Accordingly, the dried samples were also weighted repeatedly until the mass became constant (
Wd). The measurements were carried out three times each. These values correspond to the first swelling. The average data were taken for the determination of swelling ratio
S using the following equation:
where
(g) is the weight of the swollen nanofibrous mat or film and
(g) is the weight of the samples after drying at room temperature.
After drying, rehydration of dried samples was tested under wet form using the same conditions after two days in water at room temperature.
2.6.3. NMR Characterization of Nanofibers
The composition of the nanofibers in chitosan, PEO and acetic acid (or acetate) remaining in the fibers were determined by
1H NMR at 80 °C on a Bruker Avance III 400 spectrometer (Billerica, MA, USA). Selected samples were used to analyze the NMR spectrum and exemplify the change in the nanofibers compositions [
13]. Nanofiber samples of 7 to 10 mg were dissolved in 1 mL D
2O in presence of stoichiometric amount of DCl. Analysis of spectra allows the determination of the amount of acetic acid remaining in the dried samples and yield of PEO remaining in the samples after washing in different conditions.
2.6.4. Rheological Behavior
Rheological characteristics were studied using a ARG2 rheometer from TA Instruments (New Castle, DE, USA) with a cone-plate geometry; the cone has a diameter of 25 mm, a 4° angle and a 107 μm gap. The temperature is controlled at 20 °C by a Peltier plate. Steady-state flow experiments were performed in the range of 0.01 to 10 s−1.
2.6.5. Ionic Conductivity of Blend Solutions
The conductivity of solution was determined at 20 °C using a Conductimeter sensION+ EC7 from Hach Lange (Loveland, CO, USA) equipped with a titanium electrode Crison 5073. The solvent 0.5 M acetic acid has a conductivity of 1.211 mS/cm.
2.6.6. Mechanical Characterization
The measurements were carried out using ARES-G2 rheometer (TA Instruments, New Castle, DE, USA) equipped with a rectangular geometry, used for axial tension consisting of two axial clamps that hold the material when the force is applied. Samples were taken from the nanofibrous electrospun matrices or films maintaining a length/width ratio around 2.69 (suggested value in the rheometer procedure). The results are expressed as the Stress σ = Force applied/section area in Pa. In addition, to compare the samples which have not the same morphology, results of tensile tests are expressed by the reduced force in N/(kg/m2) including the mass of the sample divided by its area.
Break tests were performed using the same geometry, starting from a zero-applied force until the material presents a breaking point, with a deformation rate of 0.01 mm/s. The experiments were carried out at constant temperature around 20 °C and a special device was adopted to maintain the relative humidity in the sample environment.
The rheometer also allowed obtaining of the thickness of the samples by measuring the gap between the two plates when they approach film or fiber mat as close as possible until the detector perceives zero axial force during compression. This measurement was repeated with a micrometer (Mitutoyo Digimatic micrometer; −25 mm with precision of 0.001 mm) giving very close values. Both techniques used to determine the thickness are in good agreement with a precision of 1 μm.
The dynamic mechanical analysis Dynamic mechanical analysis (DMA) tests were performed using an initial force applied of 0.02 N with deformations between 0.01% and 0.1% strain imposed by the length/width ratio of the sample. For analysis, the storage moduli
E observed were normalized taking into consideration the sample weight per unit of surface with the expression:
4. Conclusions
In this paper, the influence of the structure of materials based on chitosan was examined. Firstly, films were casted from PEO/chitosan blends in different conditions. It was clearly shown that the degrees of swelling and mechanical properties of these chitosan films are controlled by the content and conditions of PEO addition even after extraction of PEO. The degree of swelling increases when PEO content increased up to 60% in the blend. In the same time, the specific modulus Es from DMA increases in the initial dried state as well as in the wet state after PEO extraction corresponding to a better dispersion of chitosan and stronger inter-chain interactions (involving H-bond stabilization). Such chitosan films prepared with 90% chitosan in the initial blend with PEO powder (MW = 1 × 106) and after PEO extraction have stress at beak around 6.6 MPa or 60 N/(kg/m2) with an elongation at beak larger than 40% in the wet state. These results confirm the good film forming character of chitosan recognized for a long time. The films are less brittle when casted from the blend due to their more porous and hydrophilic structure.
Secondly, using the same solutions, electrospinning was performed in experimental conditions optimized to get good nanofibers which were tested in the initial state but also after PEO extraction in the wet state. This study allows discussion of the advantage of preparing chitosan nanofibers among unstructured films. The PEO extraction is more efficient under fiber morphology as shown by NMR. The density decreases and the degree of hydration of nanofiber mats increases much when processed in presence of PEO compared with film. The ratio between Es values in the initial composite state and in the wet state on pure chitosan remains lower than on films corresponding to a larger stability of the network formed. In the same time, the specific modulus Es is much larger on the mats in the wet state than under film structure even if the density is lower. This is attributed to the larger porosity and higher dispersion and higher degree of connection between the chitosan chains. This aspect is certainly interesting for cell development. At 90% chitosan blended with 5% PEO solution (MW = 5 × 106), after extraction of PEO in the wet state, the stress at break is around 1.2 MPa or 47 N/(Kg/m2) and 32% elongation at break which are of the same order of magnitude than for films even with a lower density of fibrous material. One advantage of the nanofibers is the large porosity of membranes as well as its large specific surface allowing a possible high surface adsorption.
To conclude, for the first time, chitosan nanofibers mats are prepared and characterized completely for their structural and mechanical characteristics in the dried and wet state allowing the use of these materials for biological applications in the near future. In addition, it must be remembered that these fibers are stable in aqueous medium at pH higher than chitosan pK0 for years.