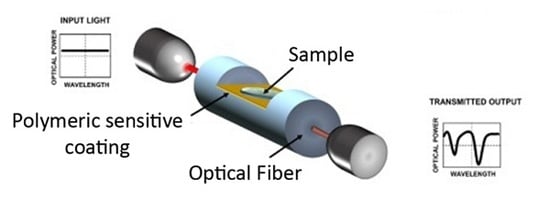
Optical Fiber Sensors Based on Polymeric Sensitive Coatings
Abstract
:1. Introduction
2. Polymers as a Support of the Sensitive Element
2.1. Immobilization of Luminescent Materials
2.2. Immobilization of Metal Nanoparticles
2.3. Immobilization of Metal Oxide Nanoparticles
3. Active Polymers for Optical Fiber Sensors
4. Final Remarks
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Peplow, M. The Plastics Revolution: How Chemists are Pushing Polymers to New Limits. Nature 2016, 536, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Majumdar, S. Polymers in Sensor Applications. Prog. Polym. Sci. (Oxf.) 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.; Yin, X.; Tong, L. Polymer Single-Nanowire Optical Sensors. Nano Lett. 2008, 8, 2757–2761. [Google Scholar] [CrossRef] [PubMed]
- Benito-Peña, E.; Carrasco, S.; Navarro-Villoslada, F.; Walt, D.R.; Moreno-Bondi, M.C. Optically-Based Molecularly Imprinted Polymers Sensors; Optics InfoBase Conference Papers; Optical Society of America: Washington, DC, USA, 2017; Volume Part F58-Sensors. [Google Scholar]
- Liu, B.; Cang, H.; Jin, J. Molecularly Imprinted Polymers Based Electrochemical Sensor for 2,4-Dichlorophenol Determination. Polymers 2016, 8, 309. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Mizuno, Y.; Nakamura, K. Brillouin Scattering in Polymer Optical Fibers: Fundamental Properties and Potential use in Sensors. Polymers 2011, 3, 886–898. [Google Scholar] [CrossRef]
- Peters, K. Polymer Optical Fiber Sensors—A Review. Smart Mater. Struct. 2011, 20, 013002. [Google Scholar] [CrossRef]
- Prado, A.R.; Leal-Junior, A.G.; Marques, C.; Leite, S.; Sena, G.L.D.; Machado, L.C.; Frizera, A.; Ribeiro, M.R.N.; Pontes, M.J. Polymethyl Methacrylate (PMMA) Recycling for the Production of Optical Fiber Sensor Systems. Opt. Express 2017, 25, 30051–30060. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Woyessa, G.; Stajanca, P.; Markos, C.; Stefani, A.; Nielsen, K.; Rasmussen, H.K.; Krebber, K.; Bang, O. Fabrication and Characterization of Polycarbonate Microstructured Polymer Optical Fibers for High-Temperature-Resistant Fiber Bragg Grating Strain Sensors. Opt. Mater. Express 2016, 6, 649–659. [Google Scholar] [CrossRef]
- Markos, C.; Stefani, A.; Nielsen, K.; Rasmussen, H.K.; Yuan, W.; Bang, O. High-Tg TOPAS Microstructured Polymer Optical Fiber for Fiber Bragg Grating Strain Sensing at 110 Degrees. Opt. Express 2013, 21, 4758–4765. [Google Scholar] [CrossRef] [PubMed]
- Blythe, A.R.; Vinson, J.R. Polymeric Materials for Devices in Optical Fibre Systems. Polym. Adv. Technol. 2000, 11, 601–611. [Google Scholar] [CrossRef]
- Del Villar, I.; Partridge, M.; Rodriguez, W.E.; Fuentes, O.; Socorro, A.B.; Diaz, S.; Corres, J.M.; James, S.W.; Tatam, R.P. Sensitivity Enhancement in Low Cutoff Wavelength Long-Period Fiber Gratings by Cladding Diameter Reduction. Sensors 2017, 17, 2094. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, J.; Hernáez, M.; Zamarreño, C.R.; Arregui, F.J. Coatings for Optical Fiber Sensors. In Comprehensive Materials Processing; Newnes: Oxford, UK, 2014; Volume 13, pp. 103–119. [Google Scholar]
- Zamarreño, C.R.; Hernáez, M.; Del Villar, I.; Matías, I.R.; Arregui, F.J. Optical Fiber pH Sensor Based on Lossy-Mode Resonances by Means of Thin Polymeric Coatings. Sens. Actuators B Chem. 2011, 155, 290–297. [Google Scholar] [CrossRef]
- Elosua, C.; Arregui, F.J.; Villar, I.D.; Ruiz-Zamarreño, C.; Corres, J.M.; Bariain, C.; Goicoechea, J.; Hernaez, M.; Rivero, P.J.; Socorro, A.B.; et al. Micro and Nanostructured Materials for the Development of Optical Fibre Sensors. Sensors 2017, 17, 2312. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Megret, P.; Caucheteur, C. Surface Plasmon Excitation at Near-Infrared Wavelengths in Polymer Optical Fibers. Opt. Lett. 2015, 40, 3998–4001. [Google Scholar] [CrossRef] [PubMed]
- Del Villar, I.; Hernaez, M.; Zamarreno, C.R.; Sánchez, P.; Fernández-Valdivielso, C.; Arregui, F.J.; Matias, I.R. Design Rules for Lossy Mode Resonance Based Sensors. Appl. Opt. 2012, 51, 4298–4307. [Google Scholar] [CrossRef] [PubMed]
- Chah, K.; Kinet, D.; Caucheteur, C. Negative Axial Strain Sensitivity in Gold-Coated Eccentric Fiber Bragg Gratings. Sci. Rep. 2016, 6, 38042. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Saez-Rodriguez, D.; Marques, C.; Bang, O.; Webb, D.J.; Mégret, P.; Caucheteur, C. Polarization Effects in Polymer FBGs: Study and use for Transverse Force Sensing. Opt. Express 2015, 23, 4581–4590. [Google Scholar] [CrossRef] [PubMed]
- Alberto, N.J.; Marques, C.A.; Pinto, J.L.; Nogueira, R.N. Three-Parameter Optical Fiber Sensor Based on a Tilted Fiber Bragg Grating. Appl. Opt. 2010, 49, 6085–6091. [Google Scholar] [CrossRef]
- Min, R.; Marques, C.; Nielsen, K.; Bang, O.; Ortega, B. Fast Inscription of Long Period Gratings in Microstructured Polymer Optical Fibers. IEEE Sens. J. 2018, 18, 1919–1923. [Google Scholar] [CrossRef]
- Larrión, B.; Hernáez, M.; Arregui, F.J.; Goicoechea, J.; Bravo, J.; Matías, I.R. Photonic Crystal Fiber Temperature Sensor Based on Quantum Dot Nanocoatings. J. Sens. 2009, 2009, 932471. [Google Scholar] [CrossRef]
- Baldini, F.; Bracci, S. Polymers for Optical Fiber Sensors; Osada, Y., De Rossi, D.E., Eds.; Polymer Sensors and Actuators; Springer: Berlin/Heidelberg, Germany, 2000; pp. 91–107. [Google Scholar]
- De Acha, N.; Elosua, C.; Matias, I.; Arregui, F.J. Luminescence-Based Optical Sensors Fabricated by Means of the Layer-by-Layer Nano-Assembly Technique. Sensors 2017, 17, 2826. [Google Scholar] [CrossRef] [PubMed]
- Elosua, C.; De Acha, N.; Lopez-Torres, D.; Matias, I.R.; Arregui, F.J. Luminescent Optical Fiber Oxygen Sensor Following Layer-By-Layer Method. Procedia Eng. 2014, 87, 987–990. [Google Scholar] [CrossRef]
- Elosua, C.; De Acha, N.; Hernaez, M.; Matias, I.R.; Arregui, F.J. Layer-by-Layer Assembly of a Water-Insoluble Platinum Complex for Optical Fiber Oxygen Sensors. Sens. Actuators B Chem. 2015, 207, 683–689. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Martínez, D.; Hernáez, M.; Matías, I.R.; Arregui, F.J. Comparative Study of Polymeric Matrices Embedding Oxygen-Sensitive Fluorophores by Means of Layer-by-Layer Nanosassembly. Sens. Actuators B Chem. 2017, 239, 1124–1133. [Google Scholar] [CrossRef]
- Chu, C.-S.; Lo, Y.-L. Highly Sensitive and Linear Calibration Optical Fiber Oxygen Sensor Based on Pt(II) Complex Embedded in Sol-Gel Matrix. Sens. Actuators B Chem. 2011, 155, 53–57. [Google Scholar] [CrossRef]
- Chu, C.-S.; Lo, Y.-L. Highly Sensitive and Linear Optical Fiber Carbon Dioxide Sensor Based on Sol-Gel Matrix Doped with Silica Particles and HPTS. Sens. Actuators B Chem. 2009, 143, 205–210. [Google Scholar] [CrossRef]
- Chu, C.-S.; Lo, Y.-L.; Sung, T.-W. Enhanced Oxygen Sensing Properties of Pt(II) Complex and Dye Entrapped Core-Shell Silica Nanoparticles Embedded in Sol-Gel Matrix. Talanta 2010, 82, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-S.; Sung, T.-W.; Lo, Y.-L. Enhanced Optical Oxygen Sensing Property Based on Pt(II) Complex and Metal-Coated Silica Nanoparticles Embedded in Sol-Gel Matrix. Sens. Actuators B Chem. 2013, 185, 287–292. [Google Scholar] [CrossRef]
- Amao, Y.; Miyashita, T.; Okura, I. Platinum Tetrakis(Pentafluorophenyl)Porphyrin Immobilized in Polytrifluoroethylmethacrylate Film as a Photostable Optical Oxygen Detection Material. J. Fluor. Chem. 2001, 107, 101–106. [Google Scholar] [CrossRef]
- Medina-Rodríguez, S.; De La Torre-Vega, A.; Fernández-Sánchez, J.F.; Fernández-Gutiérrez, A. An Open and Low-Cost Optical-Fiber Measurement System for the Optical Detection of Oxygen using a Multifrequency Phase-Resolved Method. Sens. Actuators B Chem. 2013, 176, 1110–1120. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Matías, I.R.; Arregui, F.J. Enhancement of Luminescence-Based Optical Fiber Oxygen Sensors by Tuning the Distance between Fluorophore Layers. Sens. Actuators B Chem. 2017, 248, 836–847. [Google Scholar] [CrossRef]
- Gonçalves, H.M.R.; Duarte, A.J.; Davis, F.; Higson, S.P.J.; Esteves da Silva, J.C.G. Layer-by-Layer Immobilization of Carbon Dots Fluorescent Nanomaterials on Single Optical Fiber. Anal. Chim. Acta 2012, 735, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.M.R.; Duarte, A.J.; Esteves da Silva, J.C.G. Optical Fiber Sensor for Hg(II) Based on Carbon Dots. Biosens. Bioelectron. 2010, 26, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.S.; Kaul, S.; Chinnayelka, S.; McShane, M.J. Fiber Optic Biosensors Comprising Nanocomposite Multilayered Polymer and Nanoparticle Ultrathin Films. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Cancun, Mexico, 17–21 September 2003; Volume 4, pp. 2987–2990. [Google Scholar]
- Ban, S.; Hosoki, A.; Nishiyama, M.; Seki, A.; Watanabe, K. Optical fiber oxygen sensor using layer-by-layer stacked porous composite membranes. In Proceedings of the SPIE—The International Society for Optical Engineering, San Francisco, CA, USA, 18 April 2016; Volume 9754. [Google Scholar]
- Zolkapli, M.; Saharudin, S.; Herman, S.H.; Abdullah, W.F.H. The Influence of Sol-Gel Coated Length and Withdrawal Rate on Plastic Optical Fiber Core towards Oxygen Gas Sensing Sensitivity. J. Teknol. 2016, 78, 87–91. [Google Scholar] [CrossRef]
- Mahmud, Z.; Herman, S.H.; Noor, U.M.; Saharudin, S. Performance characterization of optical fiber oxygen sensor in gas and aqueos phase. In Proceedings of the 2013 IEEE Student Conference on Research and Development, Putrajaya, Malaysia, 16–17 December 2013; pp. 569–571. [Google Scholar]
- Saharudin, S.; Isha, K.M.; Mahmud, Z.; Herman, S.H.; Noor, U.M. Performance evaluation of optical fiber sensor using different oxygen sensitive nano-materials. In Proceedings of the 2013 IEEE 4th International Conference on Photonics (ICP), Melaka, Malaysia, 28–30 October 2013; pp. 309–312. [Google Scholar]
- Matejec, V.; Mrázek, J.; Hayer, M.; Podrazký, O.; Kanka, J.; Kašík, I. Sensitivity of Microstructure Fibers to Gaseous Oxygen. Mater. Sci. Eng. C 2008, 28, 876–881. [Google Scholar] [CrossRef]
- Rigo, M.V.; Geissinger, P. Crossed Optical Fiber Sensor Arrays for High-Spatial-Resolution Sensing: Application to Dissolved Oxygen Concentration Measurements. J. Sens. 2012, 2012, 464092. [Google Scholar] [CrossRef]
- Wencel, D.; Barczak, M.; Borowski, P.; McDonagh, C. The Development and Characterisation of Novel Hybrid Sol-Gel-Derived Films for Optical pH Sensing. J. Mater. Chem. 2012, 22, 11720–11729. [Google Scholar] [CrossRef]
- Schulman, S.G.; Chen, S.; Bai, F.; Leiner, M.J.P.; Weis, L.; Wolfbeis, O.S. Dependence of the Fluorescence of Immobilized 1-Hydroxypyrene-3,6,8-Trisulfonate on Solution pH: Extension of the Range of Applicability of a pH Fluorosensor. Anal. Chim. Acta 1995, 304, 165–170. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Koller, E. Fluorimetric Assay of Hydrolases at Longwave Excitation and Emission Wavelengths with New Substrates Possessing Unique Water Solubility. Anal. Biochem. 1983, 129, 365–370. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Chu, C.-S. Highly sensitive and linear optical fiber carbon dioxide sensor based on sol-gel matrix doped with silica particles and HPTS. In Proceedings of the SPIE—The International Society for Optical Engineering, Edinburgh, UK, 5–9 October 2009; Volume 7503. [Google Scholar]
- Lo, Y.-L.; Chu, C.-S. Fiber-optic carbon dioxide sensor based on fluorinated xerogels doped with HPTS. In Proceedings of the SPIE—The International Society for Optical Engineering, Perth, Australia, 16 May 2008; Volume 7004. [Google Scholar]
- Kasik, I.; Mrazek, J.; Martan, T.; Pospisilova, M.; Podrazky, O.; Matejec, V.; Hoyerova, K.; Kaminek, M. Fiber-Optic pH Detection in Small Volumes of Biosamples. Anal. Bioanal. Chem. 2010, 398, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, J.; Ruiz Zamarreño, C.; Matías, I.R.; Arregui, F.J. Study and Optimization of the Photobleaching in Self-Assembled Optical Fiber pH Sensors Based on HPTS Using DABCO Antifading Agent; Optics InfoBase Conference Papers; Optical Society of America: Washington, DC, USA, 2006. [Google Scholar]
- Goicoechea, J.; Zamarreño, C.R.; Matias, I.R.; Arregui, F.J. Minimizing the Photobleaching of Self-Assembled Multilayers for Sensor Applications. Sens. Actuators B Chem. 2007, 126, 41–47. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Bravo, J.; Goicoechea, J.; Matias, I.R.; Arregui, F.J. Response Time Enhancement of pH Sensing Films by Means of Hydrophilic Nanostructured Coatings. Sens. Actuators B Chem. 2007, 128, 138–144. [Google Scholar] [CrossRef]
- Otte, M.A.; Sepúlveda, B.; Ni, W.; Juste, J.P.; Liz-Marzán, L.M.; Lechuga, L.M. Identification of the Optimal Spectral Region for Plasmonic and Nanoplasmonic Sensing. ACS Nano 2010, 4, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-Based Nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape Control in Gold Nanoparticle Synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Nanometals: Formation and Color. Mater. Today 2004, 7, 26–31. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Urrutia, A.; Arregui, F.J. Effect of both Protective and Reducing Agents in the Synthesis of Multicolor Silver Nanoparticles. Nanoscale Res. Lett. 2013, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Goicoechea, J.; Urrutia, A.; Matias, I.R.; Arregui, F.J. Multicolor Layer-by-Layer Films using Weak Polyelectrolyte Assisted Synthesis of Silver Nanoparticles. Nanoscale Res. Lett. 2013, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Shiratori, S.S.; Rubner, M.F. Controlling Bilayer Composition and Surface Wettability of Sequentially Adsorbed Multilayers of Weak Polyelectrolytes. Macromolecules 1998, 31, 4309–4318. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH-Dependent Thickness Behavior of Sequentially Adsorbed Layers of Weak Polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Rivero, P.J.; Hernaez, M.; Goicoechea, J.; Matías, I.R.; Arregui, F.J. A Comparative Study in the Sensitivity of Optical Fiber Refractometers Based on the Incorporation of Gold Nanoparticles into Layer-by-Layer Films. Int. J. Smart Sens. Intell. Syst. 2015, 8, 822–841. [Google Scholar] [CrossRef]
- Rivero, P.J.; Hernaez, M.; Goicoechea, J.; Matias, I.R.; Arregui, F.J. Optical fiber refractometers based on localized surface plasmon resonance (LSPR) and lossy mode resonance (LMR). In Proceedings of the SPIE—The International Society for Optical Engineering, Santander, Spain, 2 June 2014; Volume 9157. [Google Scholar]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Optical Fiber Humidity Sensors Based on Localized Surface Plasmon Resonance (LSPR) and Lossy-Mode Resonance (LMR) in Overlays Loaded with Silver Nanoparticles. Sens. Actuators B Chem. 2012, 173, 244–249. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J.; Matías, I.R. Humidity Sensor Based on Silver Nanoparticles Embedded in a Polymeric Coating. Int. J. Smart Sens. Intell. Syst. 2012, 5, 71–83. [Google Scholar] [CrossRef]
- Socorro, A.B.; Rivero, P.J.; Hernaez, M.; Goicoechea, J.; Matias, I.R.; Arregui, F.J. Optical fiber pH sensor based on gold nanoparticles into polymeric coatings. In Proceedings of the SPIE—The International Society for Optical Engineering, Barcelona, Spain, 21 May 2015; Volume 9517. [Google Scholar]
- Rivero, P.J.; Goicoechea, J.; Hernaez, M.; Socorro, A.B.; Matias, I.R.; Arregui, F.J. Optical Fiber Resonance-Based pH Sensors using Gold Nanoparticles into Polymeric Layer-by-Layer Coatings. Microsyst. Technol. 2016, 22, 1821–1829. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Matias, I.R.; Arregui, F.J. A Lossy Mode Resonance Optical Sensor using Silver Nanoparticles-Loaded Films for Monitoring Human Breathing. Sens. Actuators B Chem. 2013, 187, 40–44. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.; Yang, T.Y.; Lee, C.H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.L.; Wang, H.H.; Yeh, H.I.; et al. Synthesis, Characterization, and Bioconjugation of Fluorescent Gold Nanoclusters toward Biological Labeling Applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, A.; Goicoechea, J.; Rivero, P.J.; Pildain, A.; Arregui, F.J. Optical Fiber Sensors Based on Gold Nanorods Embedded in Polymeric Thin Films. Sens. Actuators B Chem. 2018, 255, 2105–2112. [Google Scholar] [CrossRef]
- Li, L.; Liang, Y.; Xie, L.; Lu, M.; Peng, W. Optical fiber surface plasmon resonance sensor with surface modified gold nanorods for biochemical detection. In Proceedings of the SPIE—The International Society for Optical Engineering, Beijing, China, 13 November 2014; Volume 9277. [Google Scholar]
- Shao, Y.; Xu, S.; Zheng, X.; Wang, Y.; Xu, W. Optical Fiber LSPR Biosensor Prepared by Gold Nanoparticle Assembly on Polyelectrolyte Multilayer. Sensors 2010, 10, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Yadav, P.; Gupta, B.D. Surface Plasmon Resonance Based Fiber Optic Hydrogen Peroxide Sensor using Polymer Embedded Nanoparticles. Sens. Actuators B Chem. 2013, 182, 330–335. [Google Scholar] [CrossRef]
- Satija, J.; Punjabi, N.S.; Sai, V.V.R.; Mukherji, S. Optimal Design for U-Bent Fiber-Optic LSPR Sensor Probes. Plasmonics 2014, 9, 251–260. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Mukherji, S.; Mukherji, S. Probing the Localized Surface Plasmon Field of a Gold Nanoparticle-Based Fibre Optic Biosensor. Plasmonics 2016, 11, 753–761. [Google Scholar] [CrossRef]
- Gowri, A.; Sai, V.V.R. Development of LSPR Based U-Bent Plastic Optical Fiber Sensors. Sens. Actuators B Chem. 2016, 230, 536–543. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Biswas, R. LSPR Enhanced Gasoline Sensing with a U-Bent Optical Fiber. J. Phys. D 2016, 49, 305104. [Google Scholar] [CrossRef]
- Wan, M.; Luo, P.; Jin, J.; Xing, J.; Wang, Z.; Wong, S.T.C. Fabrication of Localized Surface Plasmon Resonance Fiber Probes using Ionic Self-Assembled Gold Nanoparticles. Sensors 2010, 10, 6477–6487. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; D’Agostino, G.; Donà, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L. Localized Surface Plasmon Resonance with Five-Branched Gold Nanostars in a Plastic Optical Fiber for Bio-Chemical Sensor Implementation. Sensors 2013, 13, 14676–14686. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Bian, C.; Tong, J.-H.; Sun, J.-Z.; Xia, S.-H. A Localized Surface Plasmon Resonance DNA Biosensor Based on Gold Nanospheres Coated on the Tip of the Fiber. Optoelectron. Lett. 2016, 12, 157–160. [Google Scholar] [CrossRef]
- Guo, H.; Tao, S. Sol-Gel Synthesis of Palladium-Doped Silica Nanocomposite Fiber using Triton X-100 Micelle Template and the Application for Hydrogen Gas Sensing. IEEE Sens. J. 2007, 7, 323–328. [Google Scholar] [CrossRef]
- Chen, I.; Lin, S.-S.; Lin, T.-J.; Du, J.-K. Detection of Hydrofluoric Acid by a SiO2 Sol-Gel Coating Fiber-Optic Probe Based on Reflection-Based Localized Surface Plasmon Resonance. Sensors 2011, 11, 1907–1923. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.H.; Sun, T.; Grattan, K.T.V. LSPR Optical Fibre Sensors Based on Hollow Gold Nanostructures. Sens. Actuators B Chem. 2014, 191, 37–44. [Google Scholar] [CrossRef]
- Cao, J.; Tu, M.H.; Sun, T.; Grattan, K.T.V. Wavelength-Based Localized Surface Plasmon Resonance Optical Fiber Biosensor. Sens. Actuators B Chem. 2013, 181, 611–619. [Google Scholar] [CrossRef]
- Lepinay, S.; Staff, A.; Ianoul, A.; Albert, J. Improved Detection Limits of Protein Optical Fiber Biosensors Coated with Gold Nanoparticles. Biosens. Bioelectron. 2014, 52, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.K.; Parhi, P.; Jha, R. Graphene Oxide Encapsulated Gold Nanoparticle Based Stable Fibre Optic Sucrose Sensor. Sens. Actuators B Chem. 2015, 221, 835–841. [Google Scholar] [CrossRef]
- Muri, H.I.; Hjelme, D.R. LSPR Coupling and Distribution of Interparticle Distances between Nanoparticles in Hydrogel on Optical Fiber End Face. Sensors 2017, 17, 2723. [Google Scholar] [CrossRef] [PubMed]
- Muri, H.I.D.I.; Bano, A.; Hjelme, D.R. Interferometric and localized surface plasmon based fiber optic sensor. In Proceedings of the SPIE—Progress in Biomedical Optics and Imaging, San Francisco, CA, USA, 28 February 2017; Volume 10058. [Google Scholar]
- Muri, H.I.D.I.; Bano, A.; Hjelme, D.R. First step towards an interferometric and localized surface plasmon fiber optic sensor. In Proceedings of the 2017 25th Optical Fiber Sensors Conference (OFS), Jeju, Korea, 24–28 April 2017. [Google Scholar]
- Viegas, D.; Goicoechea, J.; Corres, J.M.; Santos, J.L.; Ferreira, L.A.; Araujo, F.M.; Matias, I.R. Humidity sensor based on a long-period fiber grating coated with a SiO2-nanospheres film. In Proceedings of the SPIE—The International Society for Optical Engineering, Perth, Australia, 23 May 2008; Volume 7004. [Google Scholar]
- Gomez, D.; Morgan, S.P.; Hayes Gill, B.R.; Korposh, S. Polymeric fibre optic sensor based on a SiO2 nanoparticle film for humidity sensing on wounds. In Proceedings of the SPIE—The International Society for Optical Engineering, Limerick, Ireland, 30 May 2016; Volume 9916. [Google Scholar]
- Gomez, D.; Morgan, S.P.; Hayes-Gill, B.R.; Correia, R.G.; Korposh, S. Polymeric Optical Fibre Sensor Coated by SiO2nanoparticles for Humidity Sensing in the Skin Microenvironment. Sens. Actuators B Chem. 2018, 254, 887–895. [Google Scholar] [CrossRef]
- Hernáez, M.; Villar, I.D.; Zamarreño, C.R.; Arregui, F.J.; Matias, I.R. Optical Fiber Refractometers Based on Lossy Mode Resonances Supported by TiO2 Coatings. Appl. Opt. 2010, 49, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Socorro, A.B.; Hernaez, M.; Del Villar, I.; Corres, J.M.; Arregui, F.J.; Matias, I.R. Single-Mode—multimode—single-Mode and Lossy Mode Resonance-Based Devices: A Comparative Study for Sensing Applications. Microsyst. Technol. 2016, 22, 1633–1638. [Google Scholar] [CrossRef]
- Hernaez, M.; Zamarreño, C.R.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Lossy mode resonances supported by TiO2-coated optical fibers. Procedia Eng. 2010, 5, 1099–1102. [Google Scholar] [CrossRef]
- Zubiate, P.; Zamarreño, C.R.; Sánchez, P.; Matias, I.R.; Arregui, F.J. High sensitive and selective C-reactive protein detection by means of lossy mode resonance based optical fiber devices. Biosens. Bioelectron. 2017, 93, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zamarreño, C.R.; Hernaez, M.; Sanchez, P.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Optical fiber humidity sensor based on lossy mode resonances supported by TiO2/PSS coatings. Procedia Eng. 2011, 25, 1385–1388. [Google Scholar] [CrossRef]
- Arregui, F.J.; Ciaurriz, Z.; Oneca, M.; Matias, I.R. An Experimental Study about Hydrogels for the Fabrication of Optical Fiber Humidity Sensors. Sens. Actuators B Chem. 2003, 96, 165–172. [Google Scholar] [CrossRef]
- Pathak, A.K.; Singh, V.K. A Wide Range and Highly Sensitive Optical Fiber pH Sensor using Polyacrylamide Hydrogel. Opt. Fiber Technol. 2017, 39, 43–48. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhang, M.; Tu, D.; Mao, X.; Liao, Y. A Relative Humidity Sensor using a Hydrogel-Coated Long Period Grating. Meas. Sci. Technol. 2007, 18, 3131–3134. [Google Scholar] [CrossRef]
- Tierney, S.; Hasle Falch, B.M.; Hjelme, D.R.; Stokke, B.T. Determination of Glucose Levels using a Functionalized Hydrogel-Optical Fiber Biosensor: Toward Continuous Monitoring of Blood Glucose in Vivo. Anal. Chem. 2009, 81, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Tierney, S.; Volden, S.; Stokke, B.T. Glucose Sensors Based on a Responsive Gel Incorporated as a Fabry-Perot Cavity on a Fiber-Optic Readout Platform. Biosens. Bioelectron. 2009, 24, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, J.; Zamarreño, C.R.; Matías, I.R.; Arregui, F.J. Study on white light optical fiber interferometry for pH sensor applications. In Proceedings of the 2007 IEEE Sensors, Atlanta, GA, USA, 28–31 October 2007; pp. 399–402. [Google Scholar] [CrossRef]
- Arregui, F.J.; Del Villar, I.; Zamarreño, C.R.; Zubiate, P.; Matias, I.R. Giant Sensitivity of Optical Fiber Sensors by Means of Lossy Mode Resonance. Sens. Actuators B Chem. 2016, 232, 660–665. [Google Scholar] [CrossRef]
- Ozcariz, A.; Zamarreño, C.R.; Zubiate, P.; Arregui, F.J. Is there a Frontier in Sensitivity with Lossy Mode Resonance (LMR) Based Refractometers? Sci. Rep. 2017, 7, 10280. [Google Scholar] [CrossRef] [PubMed]
- Zamarreño, C.R.; Hernaez, M.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Tunable Humidity Sensor Based on ITO-Coated Optical Fiber. Sens. Actuators B Chem. 2010, 146, 414–417. [Google Scholar] [CrossRef]
- Bagchi, S.; Achla, R.; Mondal, S.K. Electrospun Polypyrrole-Polyethylene Oxide Coated Optical Fiber Sensor Probe for Detection of Volatile Compounds. Sens. Actuators B Chem. 2017, 250, 52–60. [Google Scholar] [CrossRef]
- Urrutia, A.; Goicoechea, J.; Rivero, P.J.; Matías, I.R.; Arregui, F.J. Electrospun Nanofiber Mats for Evanescent Optical Fiber Sensors. Sens. Actuators B Chem. 2013, 176, 569–576. [Google Scholar] [CrossRef]
- Corres, J.M.; Garcia, Y.R.; Arregui, F.J.; Matias, I.R. Optical Fiber Humidity Sensors using PVdF Electrospun Nanowebs. IEEE Sens. J. 2011, 11, 2383–2387. [Google Scholar] [CrossRef]
- Corres, J.M.; Arregui, F.J.; Matias, I.R.; Rodriguez, Y. High sensitivity optical fiber pH sensor using poly(acrylic acid) nanofibers. In Proceedings of the IEEE Sensors, Baltimore, MD, USA, 3–6 November 2013. [Google Scholar]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-Optic Sensors Based on Surface Plasmon Resonance: A Comprehensive Review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Hassani, A.; Skorobogatiy, M. Design of the Microstructured Optical Fiber-Based Surface Plasmon Resonance Sensors with Enhanced Microfluidics. Opt. Express 2006, 14, 11616–11621. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; De Maria, L.; Chemelli, C.; Pesavento, M.; Profumo, A.; Galatus, R.; Zeni, L. Surface Plasmon Resonance Sensor in Plastic Optical Fibers. Influence of the Mechanical Support Geometry on the Performances. Convegno Nazionale Sensori 2018, 431, 135–141. [Google Scholar]
- Cennamo, N.; D’Agostino, G.; Pesavento, M.; Zeni, L. High Selectivity and Sensitivity Sensor Based on MIP and SPR in Tapered Plastic Optical Fibers for the Detection of L-Nicotine. Sens. Actuators B Chem. 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Cennamo, N.; Maria, L.D.; D’Agostino, G.; Zeni, L.; Pesavento, M. Monitoring of Low Levels of Furfural in Power Transformer Oil with a Sensor System Based on a POF-MIP Platform. Sensors (Switzerland) 2015, 15, 8499–8511. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.D.; Shrivastav, A.M.; Usha, S.P. Surface Plasmon Resonance-Based Fiber Optic Sensors Utilizing Molecular Imprinting. Sensors (Switzerland) 2016, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Fiber Optic SPR Sensor for the Detection of Melamine using Molecular Imprinting. Sens. Actuators B Chem. 2015, 212, 404–410. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Fiber Optic Profenofos Sensor Based on Surface Plasmon Resonance Technique and Molecular Imprinting. Biosens. Bioelectron. 2016, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Valero-Navarro, A.; Medina-Castillo, A.L.; Fernandez-Sanchez, J.F.; Fernandez-Gutierrez, A. Synthesis of a Novel Polyurethane-Based-Magnetic Imprinted Polymer for the Selective Optical Detection of 1-Naphthylamine in Drinking Water. Biosens. Bioelectron. 2011, 26, 4520–4525. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; D’Agostino, G.; Galatus, R.; Bibbò, L.; Pesavento, M.; Zeni, L. Sensors Based on Surface Plasmon Resonance in a Plastic Optical Fiber for the Detection of Trinitrotoluene. Sens. Actuators B Chem. 2013, 188, 221–226. [Google Scholar] [CrossRef]




















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Optical Fiber Sensors Based on Polymeric Sensitive Coatings. Polymers 2018, 10, 280. https://doi.org/10.3390/polym10030280
Rivero PJ, Goicoechea J, Arregui FJ. Optical Fiber Sensors Based on Polymeric Sensitive Coatings. Polymers. 2018; 10(3):280. https://doi.org/10.3390/polym10030280
Chicago/Turabian StyleRivero, Pedro J., Javier Goicoechea, and Francisco J. Arregui. 2018. "Optical Fiber Sensors Based on Polymeric Sensitive Coatings" Polymers 10, no. 3: 280. https://doi.org/10.3390/polym10030280
APA StyleRivero, P. J., Goicoechea, J., & Arregui, F. J. (2018). Optical Fiber Sensors Based on Polymeric Sensitive Coatings. Polymers, 10(3), 280. https://doi.org/10.3390/polym10030280