How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Starch Films
2.3. Film Characterizations
2.3.1. Film Thickness Measurements
2.3.2. Film Observations: Colour and Environmental Scanning Electron Microscopy (ESEM)
2.3.3. Fourier Transform Infrared with Attenuated Total Reflection (FTIR-ATR)
2.3.4. Thermal Properties
2.3.5. Surface Properties
2.3.6. Hydration Properties
2.3.7. Mechanical Properties
2.3.8. Water Vapor (WVP) and Oxygen (OP) Permeabilities
2.4. Statistical Analysis
3. Results and Discussion
3.1. Hydration Properties
3.2. Interactions Involved in Starch-Glycerol Films Displayed by FTIR
3.3. Effect of Plasticizer on Film Appearance, Colour, Microstructure and Thermal Stability
3.4. Wettability Properties
3.5. Influence of Both Glycerol and Water on Functional Properties: Mechanical and Barrier Efficiencies
4. Conclusions
Author Contributions
Conflicts of Interest
References
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. Effect of glycerol on the morphology of nanocomposites made from thermoplastic starch and starch nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Shield, I. Biomass Supply Chains for Bioenergy and Biorefining; Woodhead Publishing: Sawston, UK, 2016; pp. 249–269. [Google Scholar]
- Trenth, N. Corn Starch Global Industry 2018 Sales, Supply and Consumption Forecasts to 2022. Available online: www.wandtv.com (accessed on 1 March 2018).
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Makromol. 2017, 104, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, K.; Qin, G.; Chen, Y.; Peng, L.; Wan, X.; Xiao, H.; Xia, Q. Hydrogen bonding energy determined by molecular dynamics simulation and correlation to properties of thermoplastic starch films. Carbohydr. Polym. 2017, 166, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Muscat, D.; Adhikari, B.; McKnight, S.; Guo, Q.; Adhikari, B. The physicochemical characteristics and hydrophobicity of hight amylase starch-glycerol films in the presence of three natural waxes. J. Food Eng. 2010, 119, 205–219. [Google Scholar] [CrossRef]
- Ivanič, F.; Jochec-Mošková, D.; Janigová, I.; Chodák, I. Physical properties of starch plasticized by a mixture of plasticizers. Eur. Polym. J. 2017, 93, 843–849. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Xie, F.; Flanagan, B.M.; Li, M.; Sangwan, P.; Truss, R.W.; Halley, P.J.; Shamshina, J.L.; Rogers, R.D.; McNally, T. Characteristics of starch-based films plasticized by glycerol and by the ionic liquid 1-ethyl-3methylimidazolium acetate: A comparative study. Carbohydr. Polym. 2014, 111, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Bahruji, H.; Bowker, M.; Davies, P.R.; Bouleghlimat, E.; Issarapanacheewin, S. Hydrogen generation by photocatalytic reforming of potential biofuels: Polyols, cyclic alcohols, and saccharides. J. Fotochem. Fotobiol. 2018, 356, 451–456. [Google Scholar] [CrossRef]
- Tian, H.; Guo, G.; Xiang, A.; Zhong, W.H. Intermolecular interactions and microstructure of glycerol-plasticized soy protein materials at molecular and nanometer levels. Polym. Test. 2018, in press. [Google Scholar] [CrossRef]
- Vu, H.P.N.; Lumdubwong, N. Starch behaviors and mechanical properties of starch blend films with different plasticizers. Carbohydr. Polym. 2016, 154, 112–120. [Google Scholar]
- Ferreira, W.H.; Andreade, C.T. Characterization of glycerol-plasticized starch and graphene oxide extruded hybrids. Ind. Crop. Prod. 2015, 77, 684–690. [Google Scholar] [CrossRef]
- Heydari, A.; .Alemzadeh, I.; Vossoughi, M. Functional properties of biodegradable corn starch nanocomposites for food packaging applications. Mater. Des. 2013, 50, 954–961. [Google Scholar] [CrossRef]
- Abdorreza, M.N.; Cheng, L.H.; Karim, A.A. Effects of plasticizers on thermal properties and heat sealability of sago starch films. Food Hydrocol. 2011, 25, 56–60. [Google Scholar] [CrossRef]
- OlléResa, C.P.; Jagus, R.J.; Gerschenson, L.N. Effect of natamycin, nisin and glycerol on the physicochemical properties, roughness and hydrophobicity of tapioca starch edible films. Mater. Sci. Eng. C 2014, 40, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gennadios, A.; Weller, C.L.; Hanna, M.A.; Froning, G.W. Edible Coatings and Films to Improve Food Quality; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 1996. [Google Scholar]
- Sobral, P.J.; dos Santos, J.S.; Garcia, F.T. Effect of protein and plasticizer concentration in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J. Food Eng. 2005, 70, 93–100. [Google Scholar] [CrossRef]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Karbowiak, T.; Debeaufort, F.; Voilley, A. Wetting properties at the surface of iota carrageenan-based edible films. J. Colloid Interface Sci. 2006, 294, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Zisman, W.A. Contact Angle, Wettability and Adhesion; American Chemical Society: Washington, DC, USA, 1964. [Google Scholar]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effects of carbohydrate/protein ratio effects on microsctructure, barrier and sorption properties, of wheat starch-whey protein blend edible films. J. Sci. Food Agric. 2017, 97, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarenden Press: Oxford, UK, 1975. [Google Scholar]
- Gontard, N.; Guilbert, S.; Cuq, J.L. Edible wheat gluten films: Influence of the main process variables of the film properties using response surface methodology. J. Food Sci. 1994, 57, 190–195. [Google Scholar] [CrossRef]
- ASTM Standard Test Method for Tensile Properties of Thin Plastic Sheeting; Method D882-American Society for Testing and Materials; ASTM: West Conshohocken, PA, USA, 1995.
- ASTM Standard Test Methods for Water Vapor Transmission of Materials; Method E96-American Society for Testing and Materials; ASTM: West Conshohocken, PA, USA, 1980.
- Wan, Y.Z.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X.L. Mechanical, moisture adsorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos. Sci. Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Godbillot, L.; Dole, P.; Joly, C.; Rogé, B.; Mathlouthi, M. Analysis of water binding in starch plasticized films. Food Chem. 2006, 96, 380–386. [Google Scholar] [CrossRef]
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- Zhanga, Y.; Thompson, M.; Liu, Q. The effect of pea fiber and potato pulp on thermal property, surface tension, and hydrophilicity of extruded starch thermoplastics. Carbohydr. Polym. 2011, 86, 700–707. [Google Scholar] [CrossRef]
- Leung, P.; Zorrilla, C.; Recasens, F.; Smith, J.M. Hydration of isobutene in liquid-full and trickle-bed reactors. AIChE J. 1986, 32, 1839–1847. [Google Scholar] [CrossRef]
- Kibar, E.A.A.; Us, F. Thermal, mechanical and water adsorption properties of corn starch–carboxymethylcellulose/methylcellulose biodegradable films. J. Food Eng. 2013, 11, 123–131. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of type and content of binary polyoil mixtures on physical and mechanical properties of starch-based edible films. Carbohydr. Polym. 2008, 71, 269–276. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Yamashita, F.; Laurindo, J.B. Evaluation of the effects of glycerol and sorbitol concentration and water activity on the water barrier properties of cassava starch films through a solubility approach. Carbohydr. Polym. 2008, 72, 82–87. [Google Scholar] [CrossRef]
- Teodoro, A.P.; Mali, S.; Romero, N.; Carvalho, G.M. Cassava starch films containing acetylated starch nanoparticles as reinforcement: Physical and mechanical characterization. Carbohydr. Polym. 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.B.; Müller, C.M.O.; Larotonda, F.D.S.; Laurindo, J.B. Biodegradable films based on rice starch and rice flour. J. Cereal Sci. 2010, 51, 213–219. [Google Scholar] [CrossRef]
- Mali, S.; Laram, L.B.; Pereira Ramos, L.; Grossmann, M.V.E. Relationships among the composition and physicochemical properties of starches with the characteristics of their films. J. Agric. Food Chem. 2004, 52, 7720–7725. [Google Scholar] [CrossRef] [PubMed]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of glycerol on physical and mechanical properties of wheat starch edible films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films. Effect of citric acid and carboxymethyl cellulose. Ind. Crop. Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Hu, G.; Chen, J.; Gao, J. Preparation and characteristics of oxidized potato starch films. Carbohydr. Polym. 2009, 76, 291–298. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch films. Starch/Stärke 2004, 22, 348–356. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of oil lamination between plasticized starch layers on film properties. Food Chem. 2016, 195, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Basiak, E.; Galus, S.; Lenart, A. Characterisation of composite edible films based on wheat starch and whey-protein isolate. Int. J. Food Sci. Technol. 2015, 50, 372–380. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Phase transitions in starch based films containing fatty acids. Effect on water sorption and mechanical behaviour. Food Hydrocol. 2013, 30, 408–418. [Google Scholar] [CrossRef]
- Zaleska, H.; Ring, S.; Tomasik, P. Elektrosynthesis of potato starch-whey protein isolate complexes. Carbohydr. Polym. 2001, 45, 89–94. [Google Scholar] [CrossRef]
- Souza, R.C.R.; Andrade, C.T. Investigation of the gelatinization and extrusion processes of corn starch. Adv. Polym. Technol. 2002, 21, 17–24. [Google Scholar] [CrossRef]
- Guerrero, P.; Retegi, A.; Gabilondo, N.; de la Caba, K. Mechanical and thermal properties of soy protein films processed by casting and compression. J. Food Eng. 2012, 100, 145–151. [Google Scholar] [CrossRef]
- Liu, P.; Xie, F.; Li, M.; Yu, L.; Halley, P.J.; Chen, L. Phase transitions of maize starches with different amylose contents in glycerol-water systems. Carbohydr. Polym. 2011, 85, 180–187. [Google Scholar] [CrossRef]
- Suppakul, P.; Chalernsook, B.; Ratisuthawat, B.; Prapasitthi, S.; Munchukangwan, N. Empirical modelling of moisture sorption characteristics and mechanical and barrier properties of cassava flour film and their relation to plasticizing-antiplasticizing effects. LWT-Food Sci. Technol. 2013, 50, 290–297. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Microstructural characterization of yam starch films. Carbohydr. Polym. 2002, 50, 379–386. [Google Scholar] [CrossRef]
- Jagadish, R.S.; Raj, B. Properties and sorption studies of polyethylene oxide-starch blended films. Food Hydrocol. 2011, 25, 1572–1580. [Google Scholar] [CrossRef]
- Olivia, V.; López, O.V.; Ninago, M.D.; Soledad Lencina, M.M.; García, M.A.; Andreucetti, N.A.; Ciolino, A.E.; Villara, M.A. Thermoplastic starch plasticized with alginate–glycerol mixtures: Melt-processing evaluation and film properties. Carbohydr. Polym. 2015, 126, 83–90. [Google Scholar]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.M.; Park, O.O. Processabilities and mechanical properties of Surlyn-treated starch/LDPE blends. Polym. Eng. Sci. 1995, 35, 1652–1657. [Google Scholar] [CrossRef]
- Alves, V.D.; Mali, S.; Beléia, A.; Grossmann, M.V.E. Effect if glycerol and amylose enrichment on cassava starch film properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Gontard, D.; Duchaz, C.; Cuq, J.; Guilbert, S. Edible composite films of wheat gluten and lipids: Water vapor permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Chang, Y.P.; Cheah, P.B.; Seow, C.C. Plasticizing-antiplasticizing effects of water on physical properties of tapioca starch films in the glassy state. J. Food Sci. 2000, 65, 445–451. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, L.; Li, D.; Wi, Q.; Adhikari, B. Effects of high-pressure homogenization on the properties of starch-plasticizer dispersions and their films. Carbohydr. Polym. 2011, 86, 202–207. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Marx, D.B.; Hanna, M.A. Effects of extrusion temperature and plasticizers on the physical and functional properties of starch films. Starch/Stärke 2008, 60, 527–538. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water barrier of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef] [PubMed]






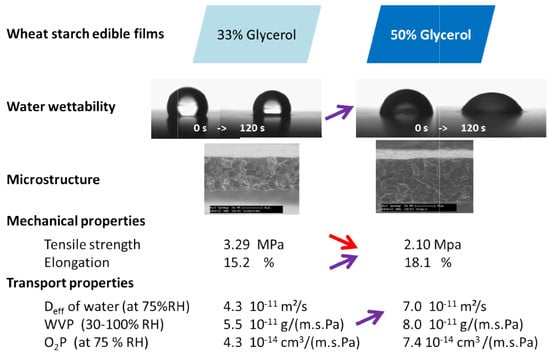
| Parameters | Wheat Starch Films | |||
|---|---|---|---|---|
| 33% Glycerol Content | 50% Glycerol Content | |||
| Thickness (µm) | 64.1 ± 8.04 a | 80.8 ± 12.59 a | ||
| Hydration characteristics | Water content (gwater 100 g−1·dm) | 3.44 ± 0.50 a | 3.72 ± 0.50 a | |
| Swelling index (%) | 38.99 ± 2.44 a | 39.20 ± 1.43 a | ||
| Solubility in water (%) | 30.16 ± 2.25 a | 34.76 ± 2.18 a | ||
| GAB parameters | m0 (gwater 100 g−1·dm) | 16.4 ± 2.2 a | 19.8 ± 1.6 b | |
| K | 0.93 ± 0.02 a | 0.97 ± 0.01 a | ||
| C | 754 ± 3 × 105 a | 105 × 109 ± 1016 a | ||
| R2 | 0.962 | 0.989 | ||
| Colour parameters | L | 95.87 ± 0.51 a | 95.48 ± 0.39 a | |
| a | −0.15 ± 0.05 a | −0.24 ± 0.06 a | ||
| b | 2.93 ± 0.19 a | 3.19 ± 0.29 a | ||
| ∆E | 0.97 ± 0.49 a | 1.46 ± 0.43 a | ||
| Surface characteristics | (mN/m) | 56.22 a | 60.28 b | |
| (mN/m) | 38.01 a | 35.40 a | ||
| (mN/m) | 18.21 a | 24.88 b | ||
| (mN/m) | 36.0 a (R2 = 0.87) | 36.0 a (R2 = 0.88) | ||
| WA (mJ/m2) | 118.5 a | 126.8 b | ||
| WC (mJ/m2) | 112.4 a | 120.5 b | ||
| WS (mJ/m2) | −6.05 a | −6.24 a | ||
| Mechanical properties | TS (MPa) | 3.29 ± 0.79 a | 2.10 ± 0.76 a | |
| YM (Mpa) | 0.12 ± 0.05 a | 0.10 ± 0.09 a | ||
| E (%) | 15.21 ± 5.88 a | 18.08 ± 5.40 a | ||
| Transfer properties | Water diffusivity (10−11 m2/s) | at 75% RH | 4.3 ± 0.9 a | 7.0 ± 0.5 b |
| Water vapour permeability (10−10 g·m−1·s−1·Pa−1) | 33–0% RH | 0.52 ± 0.04 a | 0.92 ± 0.06 b | |
| 75–30% RH | 6.05 ± 0.62 c | 8.77 ± 0.59 d | ||
| 100–30% RH | 5.48 ± 0.36 c | 8.01 ± 0.15 d | ||
| Oxygen permeability 1014 (cm3·m−1·s−1·Pa−1) | 33% RH | 3.58 ± 2.72 a | 7.23 ± 1.00 b | |
| 75% RH | 4.30 ± 0.77 a | 7.41 ± 1.39 b | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. https://doi.org/10.3390/polym10040412
Basiak E, Lenart A, Debeaufort F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers. 2018; 10(4):412. https://doi.org/10.3390/polym10040412
Chicago/Turabian StyleBasiak, Ewelina, Andrzej Lenart, and Frédéric Debeaufort. 2018. "How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films" Polymers 10, no. 4: 412. https://doi.org/10.3390/polym10040412
APA StyleBasiak, E., Lenart, A., & Debeaufort, F. (2018). How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers, 10(4), 412. https://doi.org/10.3390/polym10040412