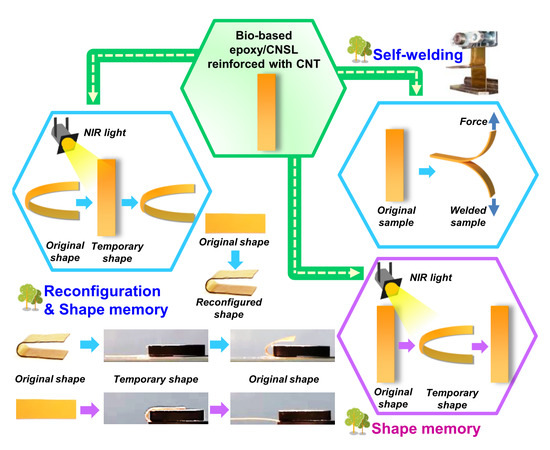
Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
2.2.1. Evaluation of Curing Reaction
2.2.2. Analysis of Thermomechanical Properties
2.2.3. Shape Memory Phenomena Induced by NIR
2.2.4. Stress Relaxation Behavior
2.2.5. Self-Welding Properties
2.2.6. Scanning Electron Microscopy (SEM) Observation
3. Results and Discussion
3.1. Curing Reaction of EPN/CNSL Composites
3.2. Thermomechanical Properties of EPN/CNSL Film Composites
3.3. Shape Memory Effect of EPN/CNSL Composites
3.4. Shape Memory Behavior of EPN/CNSL Composites Induced by NIR Irradiation
3.5. Stress Relaxation of EPN/CNSL Composites
3.6. Reconfiguration and Shape Memory of EPN/CNSL Composites
3.7. Self-Welding of EPN/CNSL Composites
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dong, Y.; Ni, Q.-Q.; Fu, Y. Preparation and characterization of water-borne epoxy shape memory composites containing silica. Compos. Part A 2015, 72, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, R.; Ye, S.; Ge, Y.; Tu, Y.; Yang, X. Graphene oxide/poly(n-isopropylacrylamide) hybrid film-based near-infrared light-driven bilayer actuators with shape memory effect. Sens. Actuators B Chem. 2018, 255, 2971–2978. [Google Scholar] [CrossRef]
- Lu, H.; Yao, Y.; Huang, W.M.; Leng, J.; Hui, D. Significantly improving infrared light-induced shape recovery behavior of shape memory polymeric nanocomposite via a synergistic effect of carbon nanotube and boron nitride. Compos. Part B 2014, 62, 256–261. [Google Scholar] [CrossRef]
- Osicka, J.; Ilčíková, M.; Mrlik, M.; Minařík, A.; Pavlinek, V.; Mosnáček, J. The impact of polymer grafting from a graphene oxide surface on its compatibility with a pdms matrix and the light-induced actuation of the composites. Polymers 2017, 9, 264. [Google Scholar] [CrossRef]
- Ilčíková, M.; Mrlík, M.; Sedláček, T.; Chorvát, D.; Krupa, I.; Šlouf, M.; Koynov, K.; Mosnáček, J. Viscoelastic and photo-actuation studies of composites based on polystyrene-grafted carbon nanotubes and styrene-b-isoprene-b-styrene block copolymer. Polymer 2014, 55, 211–218. [Google Scholar] [CrossRef]
- Yi, D.H.; Yoo, H.J.; Mahapatra, S.S.; Kim, Y.A.; Cho, J.W. The synergistic effect of the combined thin multi-walled carbon nanotubes and reduced graphene oxides on photothermally actuated shape memory polyurethane composites. J. Colloid Interface Sci. 2014, 432, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhong, J.; Meng, J.; Xian, G. The reinforcement efficiency of carbon nanotubes/shape memory polymer nanocomposites. Compos. Part B 2013, 44, 508–516. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Z.; Xu, L.; Shao, Z. Preparation and characterization of highly aligned carbon nanotubes/ polyacrylonitrile composite nanofibers. Polymers 2017, 9, 1. [Google Scholar] [CrossRef]
- Jia, X.-L.; Zhang, Q.; Huang, J.-Q.; Zheng, C.; Qian, W.-Z.; Wei, F. The direct dispersion of granular agglomerated carbon nanotubes in bismaleimide by high pressure homogenization for the production of strong composites. Powder Technol. 2012, 217, 477–481. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Fu, X.; Liu, Z.; Kong, W.; Zhou, C.; Lei, J. Multifunctional polyurethane-vitrimers completely based on transcarbamoylation of carbamates: Thermally-induced dual-shape memory effect and self-welding. RSC Adv. 2017, 7, 26858–26866. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Surface engineering of nanosilica for vitrimer composites. Compos. Sci. Technol. 2018, 154, 18–27. [Google Scholar] [CrossRef]
- Imbernon, L.; Norvez, S.; Leibler, L. Stress relaxation and self-adhesion of rubbers with exchangeable links. Macromolecules 2016, 49, 2172–2178. [Google Scholar] [CrossRef]
- Demongeot, A.; Mougnier, S.; Okada, S.; Soulié-Ziakovic, C.; Tournilhac, F. Coordination and catalysis of Zn2+ in epoxy-based vitrimers. Polym. Chem. 2016, 7, 4486–4493. [Google Scholar] [CrossRef]
- Altuna, F.; Hoppe, C.; Williams, R. Shape memory epoxy vitrimers based on dgeba crosslinked with dicarboxylic acids and their blends with citric acid. RSC Adv. 2016, 6, 88647–88655. [Google Scholar] [CrossRef]
- Belmonte, A.; Russo, C.; Ambrogi, V.; Fernández-Francos, X.; De la Flor, S. Epoxy-based shape-memory actuators obtained via dual-curing of off-stoichiometric “thiol-epoxy” mixtures. Polymers 2017, 9, 113. [Google Scholar] [CrossRef]
- Liu, W.; Fei, M.-E.; Ban, Y.; Jia, A.; Qiu, R. Preparation and evaluation of green composites from microcrystalline cellulose and a soybean-oil derivative. Polymers 2017, 9, 541. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Pizzi, A.; Abdulkhani, A.; Shakeri, A. Analysis and testing of bisphenol a-free bio-based tannin epoxy-acrylic adhesives. Polymers 2016, 8, 143. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Moradi, Y.; Ahmadi, M.; Amiri, S.; Naebe, M. Catalyzed synthesis and characterization of a novel lignin-based curing agent for the curing of high-performance epoxy resin. Polymers 2017, 9, 266. [Google Scholar] [CrossRef]
- Mathew, G.; Rhee, J.; Hwang, B.; Nah, C. Cure behavior of epoxy resin containing castor oil and cashew nut shell liquid and its derivative. J. Appl. Polym. Sci. 2007, 106, 178–184. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Neramittagapong, A.; Chindaprasirt, P. Curing kinetic, thermal and adhesive properties of epoxy resin cured with cashew nut shell liquid. Thermochim. Acta 2015, 600, 20–27. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Liu, Y.; Leng, J.; Bhattacharyya, D. Electrical actuation properties of reduced graphene oxide paper/epoxy-based shape memory composites. Compos. Sci. Technol. 2015, 106, 20–24. [Google Scholar] [CrossRef]
- ASTM D1876 (2015). Standard Test Method for Peel Resistance of Adhesives (T-Peel Test), Astm International, west conshohocken, pa. 2015. Available online: www.Astm.Org (accessed on 1 March 2018).
- Puglia, D.; Rastin, H.; Saeb, M.R.; Shojaei, B.; Formela, K. Cure kinetics of epoxy/mwcnts nanocomposites: Isothermal calorimetric and rheological analyses. Prog. Org. Coat. 2017, 108, 75–83. [Google Scholar]
- Yang, K.; Gu, M.; Jin, Y. Cure behavior and thermal stability analysis of multiwalled carbon nanotube/epoxy resin nanocomposites. J. Appl. Polym. Sci. 2008, 110, 2980–2988. [Google Scholar] [CrossRef]
- Xie, H.; Liu, B.; Sun, Q.; Yuan, Z.; Shen, J.; Cheng, R. Cure kinetic study of carbon nanofibers/epoxy composites by isothermal dsc. J. Appl. Polym. Sci. 2005, 96, 329–335. [Google Scholar] [CrossRef]
- Mordina, B.; Tiwari, R. Thermal and mechanical behavior of poly(vinyl butyral)-modified novolac epoxy/multiwalled carbon nanotube nanocomposites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Mwaikambo, L.; Ansell, M. Cure characteristics of alkali catalysed cashew nut shell liquid-formaldehyde resin. J. Mater. Sci. 2001, 36, 3693–3698. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, P.; Ren, R.; Lu, F.; Yu, Q. Cure mechanism and thermal properties of the phthalide- containing bismaleimide/epoxy system. Thermochim. Acta 2013, 559, 52–58. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Hiziroglu, S.; Rimdusit, S. Effect of cashew nut shell liquid on gelation, cure kinetics, and thermomechanical properties of benzoxazine resin. Thermochim. Acta 2011, 520, 84–92. [Google Scholar] [CrossRef]
- Dušek, K. Network formation in curing of epoxy resins. In Epoxy Resins and Composites III; Springer: Berlin, Germany, 1986; pp. 1–59. [Google Scholar]
- Ma, P.C.; Kim, J.-K.; Tang, B.Z. Effects of silane functionalization on the properties of carbon nanotube/epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 2965–2972. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Tan, S.G.; Ahmad, Z.; Chow, W.S. Interpenetrating polymer network structured thermosets prepared from epoxidized soybean oil/diglycidyl ether of bisphenol a. Polym. Int. 2014, 63, 273–279. [Google Scholar] [CrossRef]
- Puchot, L.; Verge, P.; Peralta, S.; Habibi, Y.; Vancaeyzeele, C.; Vidal, F. Elaboration of bio-epoxy/benzoxazine interpenetrating polymer networks: A composition-to-morphology mapping. Polym. Chem. 2018, 9, 472–481. [Google Scholar] [CrossRef]
- Montazeri, A.; Montazeri, N. Viscoelastic and mechanical properties of multi walled carbon nanotube/epoxy composites with different nanotube content. Mater. Des. 2011, 32, 2301–2307. [Google Scholar] [CrossRef]
- Prasad, H.C.; Hashmi, S.; Naik, A.; Bhargaw, H.N. Improved shape memory effects in multiwalled-carbon-nano-tube reinforced thermosetting polyurethane composites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Gu, S.; Yan, B.; Liu, L.; Ren, J. Carbon nanotube–polyurethane shape memory nanocomposites with low trigger temperature. Eur. Polym. J. 2013, 49, 3867–3877. [Google Scholar] [CrossRef]
- Deka, H.; Karak, N.; Kalita, R.D.; Buragohain, A.K. Biocompatible hyperbranched polyurethane/multi-walled carbon nanotube composites as shape memory materials. Carbon 2010, 48, 2013–2022. [Google Scholar] [CrossRef]
- Ni, Q.-Q.; Zhang, C.-S.; Fu, Y.; Dai, G.; Kimura, T. Shape memory effect and mechanical properties of carbon nanotube/shape memory polymer nanocomposites. Compos. Struct. 2007, 81, 176–184. [Google Scholar] [CrossRef]
- Li, G.; Meng, H. Recent Advances in Smart Self-Healing Polymers and Composites, 1st ed.; Woodhead Publishing: Waltham, MA, USA, 2015; pp. 303–305. [Google Scholar]
- Gardea, F.; Lagoudas, D.C. Characterization of electrical and thermal properties of carbon nanotube/epoxy composites. Compos. Part B 2014, 56, 611–620. [Google Scholar] [CrossRef]
- Rimdusit, S.; Lohwerathama, M.; Hemvichian, K.; Kasemsiri, P.; Dueramae, I. Shape memory polymers from benzoxazine-modified epoxy. Smart Mater. Struct. 2013, 22, 075033. [Google Scholar] [CrossRef]
- Altuna, F.I.; Pettarin, V.; Williams, R.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic control of the vitrimer glass transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- Lawton, M.I.; Tillman, K.R.; Mohammed, H.S.; Kuang, W.; Shipp, D.A.; Mather, P.T. Anhydride-based reconfigurable shape memory elastomers. ACS Macro Lett. 2016, 5, 203–207. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Kawashita, L.; Moore, D.; Williams, J.G. Protocols for the measurement of adhesive fracture toughness by peel tests. J. Adhes. 2006, 82, 973–995. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Li, H.; Jabour, J.; Yang, H.; Dunn, M.L.; Wang, T.; Qi, H.J. Interfacial welding of dynamic covalent network polymers. J. Mech. Phys. Solids 2016, 94, 1–17. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Kuang, X.; Mu, X.; Dunn, C.K.; Dunn, M.L.; Wang, T.; Qi, H.J. Recyclable 3D printing of vitrimer epoxy. Mater. Horiz. 2017, 4, 598–607. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.; Qi, H.J. Solvent assisted pressure-free surface welding and reprocessing of malleable epoxy polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]










| CNT Content (wt %) | Shape recover ratio (%) at N = 1–5 | Recovery time (s) at N = 1–5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 0 | 98.85 | 99.56 | 99.98 | 98.89 | 99.51 | 230 | 235 | 235 | 230 | 230 |
| 0.1 | 99.52 | 98.73 | 99.67 | 99.56 | 99.63 | 100 | 90 | 105 | 115 | 120 |
| 0.3 | 98.87 | 99.13 | 98.22 | 99.82 | 99.56 | 95 | 90 | 85 | 90 | 80 |
| 0.5 | 99.45 | 99.78 | 98.32 | 98.67 | 99.67 | 65 | 60 | 70 | 65 | 55 |
| CNT Content (wt %) | Original shape | Reconfigured shape | Reconfigured shape at 150 °C | Shape memory behavior activated by NIR | |
|---|---|---|---|---|---|
| Permanent SHAPE | Recovery shape | ||||
| 0 |  |  |  |  |  |
| 0.1 |  |  |  |  |  |
| 0.3 |  |  |  |  |  |
| 0.5 |  |  |  |  |  |
| CNT Content (wt %) | Shape recover ratio (%) at N = 1–5 | Recovery time (s) at N = 1–5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 0 | 96.35 | 97.87 | 88.54 | 86.33 | 85.26 | 390 | 370 | 410 | 500 | 510 |
| 0.1 | 84.18 | 77.73 | 77.53 | 72.24 | 71.36 | 170 | 210 | 190 | 180 | 190 |
| 0.3 | 79.75 | 74.47 | 70.34 | 67.28 | 67.48 | 120 | 110 | 130 | 140 | 140 |
| 0.5 | 59.73 | 57.39 | 59.37 | 53.43 | 48.17 | 80 | 40 | 40 | 40 | 40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasemsiri, P.; Lorwanishpaisarn, N.; Pongsa, U.; Ando, S. Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes. Polymers 2018, 10, 482. https://doi.org/10.3390/polym10050482
Kasemsiri P, Lorwanishpaisarn N, Pongsa U, Ando S. Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes. Polymers. 2018; 10(5):482. https://doi.org/10.3390/polym10050482
Chicago/Turabian StyleKasemsiri, Pornnapa, Narubeth Lorwanishpaisarn, Uraiwan Pongsa, and Shinji Ando. 2018. "Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes" Polymers 10, no. 5: 482. https://doi.org/10.3390/polym10050482
APA StyleKasemsiri, P., Lorwanishpaisarn, N., Pongsa, U., & Ando, S. (2018). Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes. Polymers, 10(5), 482. https://doi.org/10.3390/polym10050482