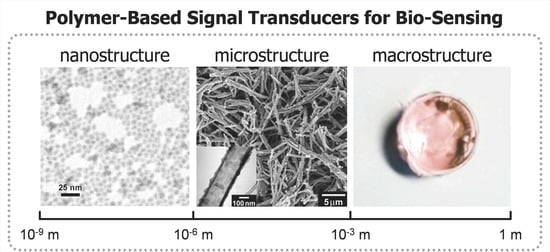
Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale
Abstract
:1. Introduction
2. Nanostructure: Nanoparticles
2.1. Physical Doping
2.2. Chemical Structure Modification of Monomers
2.3. Copolymerization
2.4. Post-Polymerization Modification
3. Macrostructure: Hydrogels
3.1. Covalent Chemistry for Hydrogels
3.1.1. Radical Polymerization
3.1.2. Other Covalent Reactions
3.2. Non-Covalent Chemistry for Hydrogels
3.2.1. Coordination Bonds
3.2.2. Supramolecular Chemistry
3.3. Miscellaneous Approaches
3.3.1. Additives
3.3.2. Molecular Imprinting
3.3.3. Multilayered Structure
3.3.4. Electrospinning
3.3.5. Hydrothermal Process
4. Summary and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Park, S.J.; Kwon, O.S.; Lee, J.E.; Jang, J.; Yoon, H. Conducting Polymer-Based Nanohybrid Transducers: A Potential Route to High Sensitivity and Selectivity Sensors. Sensors 2014, 14, 3604–3630. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, J. Conducting Polymer Nanomaterials for High-Performance Sensor Applications: Issues and Challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Zarzar, L.D.; Aizenberg, J. Stimuli-Responsive Chemomechanical Actuation: A Hybrid Materials Approach. Acc. Chem. Res. 2014, 47, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Yoon, H.; Hong, J.Y.; Jang, J. Charge-Transport Behavior in Shape-Controlled Poly(3,4-ethylenedioxythiophene) Nanomaterials: Intrinsic and Extrinsic Factors. Small 2007, 3, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Chang, M.; Jang, J. Formation of 1D Poly(3,4-ethylenedioxythiophene) Nanomaterials in Reverse Microemulsions and Their Application to Chemical Sensors. Adv. Funct. Mater. 2007, 17, 431–436. [Google Scholar] [CrossRef]
- Yoon, H.; Chang, M.; Jang, J. Sensing Behaviors of Polypyrrole Nanotubes Prepared in Reverse Microemulsions: Effects of Transducer Size and Transduction Mechanism. J. Phys. Chem. B 2006, 110, 14074–14077. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-K.; Kim, S.; Yoon, H.; Jang, J. Shape-Dependent Cytotoxicity and Proinflammatory Response of Poly(3,4-ethylenedioxythiophene) Nanomaterials. Small 2010, 6, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Dong, R.; Gordiichuk, P.; Zhang, T.; Zhuang, X.; Mai, Y.; Liu, F.; Herrmann, A.; Feng, X. Two-Dimensional Mesoscale-Ordered Conducting Polymers. Angew. Chem. Int. Ed. 2016, 55, 12516–12521. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, F.; Dong, R.; Zhang, T.; Zhang, J.; Zhuang, X.; Mai, Y.; Feng, X. Dual-Template Synthesis of 2D Mesoporous Polypyrrole Nanosheets with Controlled Pore Size. Adv. Mater. 2016, 28, 8365–8370. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gordiichuk, P.; Wu, Z.S.; Liu, Z.; Wei, W.; Wagner, M.; Mohamed-Noriega, N.; Wu, D.; Mai, Y.; Herrmann, A.; et al. Patterning Two-Dimensional Free-Standing Surfaces with Mesoporous Conducting Polymers. Nat. Commun. 2015, 6, 8817. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Yoon, H. Recent Advances in Nanostructured Conducting Polymers: from Synthesis to Practical Applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef]
- Yoon, H.; Choi, M.; Lee, K.J.; Jang, J. Versatile Strategies for Fabricating Polymer Nanomaterials with Controlled Size and Morphology. Macromol. Res. 2008, 16, 85–102. [Google Scholar] [CrossRef]
- Molino, P.J.; Yue, Z.; Zhang, B.; Tibbens, A.; Liu, X.; Kapsa, R.M.I.; Higgins, M.J.; Wallace, G.G. Influence of Biodopants on PEDOT Biomaterial Polymers: Using QCM-D to Characterize Polymer Interactions with Proteins and Living Cells. Adv. Mater. Interfaces 2014, 1, 1300122. [Google Scholar] [CrossRef]
- Park, S.J.; Song, H.S.; Kwon, O.S.; Chung, J.H.; Lee, S.H.; An, J.H.; Ahn, S.R.; Lee, J.E. Human Dopamine Receptor Nanovesicles for Gate-Potential Modulators in High-Performance Field-Effect Transistor Biosensors. Sci. Rep. 2014, 4, 4342. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Lee, S.H.; Kim, T.H.; Park, J.; Song, H.S.; Park, T.H.; Hong, S. Nanovesicle-Based Bioelectronic Nose Platform Mimicking Human Olfactory Signal Transduction. Biosens. Bioelectron. 2012, 35, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Brewer, G.J.; Wheeler, B.C. A Modified Microstamping Technique Enhances Polylysine Transfer and Neuronal Cell Patterning. Biomaterials 2003, 24, 2863–2870. [Google Scholar] [CrossRef]
- Gilmore, K.J.; Kita, M.; Han, Y.; Gelmi, A.; Higgins, M.J.; Moulton, S.E.; Clark, G.M.; Kapsa, R.; Wallace, G.G. Skeletal Muscle Cell Proliferation and Differentiation on Polypyrrole Substrates Doped with Extracellular Matrix Components. Biomaterials 2009, 30, 5292–5304. [Google Scholar] [CrossRef] [PubMed]
- Harman, D.G.; Gorkin, R., III; Stevens, L.; Thompson, B.; Wagner, K.; Weng, B.; Chung, J.H.Y. Poly(3,4-ethylenedioxythiophene):Dextran Sulfate (PEDOT:DS)–A Highly Processable Conductive Organic Biopolymer. Acta Biomater. 2015, 14, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.-L.; Kuramoto, N. Synthesis of Helical Polyanilines Using Chondroitin Sulfate as a Molecular Template. Macromol. Chem. Phys. 2004, 205, 1744–1751. [Google Scholar] [CrossRef]
- Collier, J.H.; Camp, J.P.; Hudson, T.W.; Schmidt, C.E. Synthesis and Characterization of Polypyrrole–Hyaluronic Acid Composite Biomaterials for Tissue Engineering Applications. J. Biomed. Mater. Res. 2000, 50, 574–584. [Google Scholar] [CrossRef]
- Stewart, E.M.; Liu, X.; Clark, G.M.; Kapsa, R.M.I.; Wallace, G.G. Inhibition of Smooth Muscle Cell Adhesion and Proliferation on Heparin-Doped Polypyrrole. Acta Biomater. 2012, 8, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.-L.; Kuramoto, N. Synthesis and Chiroptical Properties of Optically Active Poly(N-alkylanilines) Doped and Intertwined with Dextran Sulfate in Aqueous Solution. Macromolecules 2003, 36, 7939–7945. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Mancuso, R.; Gabriele, B. Recent Advances in the Synthesis of Thiophene Derivatives by Cyclization of Functionalized Alkynes. Molecules 2014, 19, 15687–15719. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Foot, P.J.S.; Brown, J.W.; Spearman, P. A Urea Potentiometric Biosensor Based on a Thiophene Copolymer. Biosensors 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, N.; Chan, E.; Baek, P.; Barker, D.; Williams, D.E.; Travas-Sejdic, J. New Immobilisation Method for Oligonucleotides on Electrodes Enables Highly-Sensitive, Electrochemical Label-Free Gene Sensing. Biosens. Bioelectron. 2017, 97, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yoon, H. Formation Mechanism of Conducting Polypyrrole Nanotubes in Reverse Micelle Systems. Langmuir 2005, 21, 11484–11489. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yoon, H. Facile Fabrication of Polypyrrole Nanotubes Using Reverse Microemulsion Polymerization. Chem. Commun. 2003, 6, 720–721. [Google Scholar] [CrossRef]
- Jang, J.; Chang, M.; Yoon, H. Chemical Sensors Based on Highly Conductive Poly(3,4-ethylenedioxythiophene) Nanorods. Adv. Mater. 2005, 17, 1616–1620. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, J.-H.; Lee, N.; Kim, B.-G.; Jang, J. A Novel Sensor Platform Based on Aptamer-Conjugated Polypyrrole Nanotubes for Label-Free Electrochemical Protein Detection. ChemBioChem 2008, 9, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Ahn, S.R.; Park, S.J.; Song, H.S.; Lee, S.H.; Lee, J.S.; Hong, J.-Y.; Lee, J.S.; You, S.A.; Yoon, H.; et al. Ultrasensitive and Selective Recognition of Peptide Hormone Using Close-Packed Arrays of hPTHR-Conjugated Polymer Nanoparticles. ACS Nano 2012, 6, 5549–5558. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, S.H.; Kwon, O.S.; Song, H.S.; Oh, E.H.; Park, T.H.; Jang, J. Polypyrrole Nanotubes Conjugated with Human Olfactory Receptors: High-Performance Transducer for FET-Type Bioelectronic Nose. Angew. Chem. Int. Ed. 2009, 48, 2755–2758. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Ko, S.; Jang, J. Field-Effect-Transistor Sensor Based on Enzyme-Functionalized Polypyrrole Nanotubes for Glucose Detection. J. Phys. Chem. B 2008, 112, 9992–9997. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, J. A Field-Effect-Transistor Sensor Based on Polypyrrole Nanotubes Coupled with Heparin for Thrombin Detection. Mol. Cryst. Liq. Cryst. 2008, 491, 21–31. [Google Scholar] [CrossRef]
- Boaen, N.K.; Hillmyer, M.A. Post-Polymerization Functionalization of Polyolefins. Chem. Soc. Rev. 2005, 34, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.-A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Galvin, C.J.; Genzer, J. Applications of Surface-Grafted Macromolecules Derived from Post-Polymerization Modification Reactions. Prog. Polym. Sci. 2012, 37, 871–906. [Google Scholar] [CrossRef]
- Theato, P. Functional Polymers by Post-Polymerization Modification; Klok, H.-A., Ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Kempe, K.; Hoogenboom, R.; Jaeger, M.; Schubert, U.S. Three-Fold Metal-Free Efficient (“Click”) Reactions onto a Multifunctional Poly(2-oxazoline) Designer Scaffold. Macromolecules 2011, 44, 6424–6432. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, C.; Wooley, K.L. Cycloalkenyl-Functionalized Polymers and Block Copolymers: Syntheses via Selective RAFT Polymerizations and Demonstration of Their Versatile Reactivity. Macromolecules 2009, 42, 1565–1573. [Google Scholar] [CrossRef]
- Bulmus, V.; Woodward, M.; Lin, L.; Murthy, N.; Stayton, P.; Hoffman, A. A New pH-Responsive and Glutathione-Reactive, Endosomal Membrane-Disruptive Polymeric Carrier for Intracellular Delivery of Biomolecular Drugs. J. Control. Release 2003, 93, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Boyer, C.; Jia, Z.; Zareie, H.M.; Davis, T.P.; Bulmus, V. Synthesis of Versatile Thiol-Reactive Polymer Scaffolds via RAFT Polymerization. Biomacromolecules 2008, 9, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.A.; Na, K.; Bae, Y.H. pH-Responsive Sulfonamide/PEI System for Tumor Specific Gene Delivery: An in Vitro Study. Biomacromolecules 2006, 7, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Barbey, R.; Klok, H.-A. Room Temperature, Aqueous Post-Polymerization Modification of Glycidyl Methacrylate-Containing Polymer Brushes Prepared via Surface-Initiated Atom Transfer Radical Polymerization. Langmuir 2010, 26, 18219–18230. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.D.; Shin, J.; Hoyle, C.E.; McCormick, C.L. Direct RAFT Polymerization of an Unprotected Isocyanate-Containing Monomer and Subsequent Structopendant Functionalization Using “Click”-Type Reactions. Polym. Chem. 2010, 1, 213–220. [Google Scholar] [CrossRef]
- Rabuka, D.; Parthasarathy, R.; Lee, G.S.; Chen, X.; Groves, J.T.; Bertozzi, C.R. Hierarchical Assembly of Model Cell Surfaces: Synthesis of Mucin Mimetic Polymers and Their Display on Supported Bilayers. J. Am. Chem. Soc. 2007, 129, 5462–5471. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-P.; Cai, Z.-H.; Liang, H.; Lu, J. Amphiphilic Block Copolymers with Aldehyde and Ferrocene-Functionalized Hydrophobic Block and Their Redox-Responsive Micelles. J. Mater. Chem. 2010, 20, 8375–8381. [Google Scholar] [CrossRef]
- Desai, A.; Atkinson, N.; Rivera, F., Jr.; Devonport, W.; Rees, I.; Branz, S.E.; Hawker, C.J. Hybrid Dendritic–Linear Graft Copolymers: Steric Considerations in “Coupling to” Approach. J. Polym. Sci. A 2000, 38, 1033–1044. [Google Scholar] [CrossRef]
- Šubr, V.; Ulbrich, K. Synthesis and Properties of New N-(2-hydroxypropyl)methacrylamide Copolymers Containing Thiazolidine-2-thione Reactive Groups. React. Funct. Polym. 2006, 66, 1525–1538. [Google Scholar] [CrossRef]
- Hwang, J.; Li, R.C.; Maynard, H.D. Well-Defined Polymers with Activated Ester and Protected Aldehyde Side Chains for Bio-functionalization. J. Control. Release 2007, 122, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Liotta, C.L.; Collard, D.M.; Schiraldi, D.A. Cross-Linking and Modification of Poly(ethylene terephthalate-co-2,6-anthracenedicarboxylate) by Diels−Alder Reactions with Maleimides. Macromolecules 1999, 32, 5786–5792. [Google Scholar] [CrossRef]
- Canadell, J.; Fischer, H.; De With, G.; van Benthem, R.A.T.M. Stereoisomeric Effects in Thermo-Remendable Polymer Networks Based on Diels–Alder Crosslink Reactions. J. Polym. Sci. A 2010, 48, 3456–3467. [Google Scholar] [CrossRef]
- Ohsawa, S.; Morino, K.; Sudo, A.; Endo, T. Synthesis of a Reactive Polyester Bearing α,β-Unsaturated Ketone Groups by Anionic Alternating Copolymerization of Epoxide and Bicyclic Bis(γ-butyrolactone) Bearing Isopropenyl Group. Macromolecules 2011, 44, 1814–1820. [Google Scholar] [CrossRef]
- Yang, S.K.; Weck, M. Covalent and Orthogonal Multi-functionalization of Terpolymers. Soft Matter 2009, 5, 582–585. [Google Scholar] [CrossRef]
- Wang, R.; Chen, W.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. Unprecedented Access to Functional Biodegradable Polymers and Coatings. Macromolecules 2011, 44, 6009–6016. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Xie, Z.; Zheng, Z. 3D-patterned Polymer Brush Surfaces. Nanoscale 2011, 3, 4929–4939. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S.; Brittain, W.J. Polymer Brush Grafted from an Allylsilane-Functionalized Surface. Macromolecules 2006, 39, 5675–5678. [Google Scholar] [CrossRef]
- Huang, C.-F. Surface-Initiated Atom Transfer Radical Polymerization for Applications in Sensors, Non-Biofouling Surfaces and Adsorbents. Polym. J. 2016, 48, 341–350. [Google Scholar] [CrossRef]
- Brown, A.A.; Azzaroni, O.; Fidalgo, L.M.; Huck, W.T.S. Polymer Brush Resist for Responsive Wettability. Soft Matter 2009, 5, 2738–2745. [Google Scholar] [CrossRef]
- Ma, H.; Hyun, J.; Stiller, P.; Chilkoti, A. “Non-Fouling” Oligo(ethylene glycol)—Functionalized Polymer Brushes Synthesized by Surface-Initiated Atom Transfer Radical Polymerization. Adv. Mater. 2004, 16, 338–341. [Google Scholar] [CrossRef]
- Hucknall, A.; Rangarajan, S.; Chilkoti, A. In Pursuit of Zero: Polymer Brushes that Resist the Adsorption of Proteins. Adv. Mater. 2009, 21, 2441–2446. [Google Scholar] [CrossRef]
- Ma, H.; Li, D.; Sheng, X.; Zhao, B.; Chilkoti, A. Protein-Resistant Polymer Coatings on Silicon Oxide by Surface-Initiated Atom Transfer Radical Polymerization. Langmuir 2006, 22, 3751–3756. [Google Scholar] [CrossRef] [PubMed]
- Tugulu, S.; Klok, H. Stability and Nonfouling Properties of Poly(poly(ethylene glycol) methacrylate) Brushes under Cell Culture Conditions. Biomacromolecules 2008, 9, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Tsarevsky, N.V. “Green” Atom Transfer Radical Polymerization: From Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar]
- Cao, Z.; Gordiichuk, P.I.; Loos, K.; Sudhölter, E.J.R.; de Smet, L.C.P.M. The Effect of Guanidinium Functionalization on the Structural Properties and Anion Affinity of Polyelectrolyte Multilayers. Soft Matter 2016, 12, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.R.; Chaney, B.N.; Rowley, J.; Liebmann-Vinson, A.; Genzer, J. Tailoring Cell Adhesion Using Surface-Grafted Polymer Gradient Assemblies. Adv. Mater. 2005, 17, 2802–2807. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Bobin, J. Phosphorus-Containing Polymers: A Great Opportunity for the Biomedical Field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Terayama, Y.; Yamaguchi, H.; Terada, M.; Murakami, D.; Ishihara, K.; Takahara, A. Wettability and Antifouling Behavior on the Surfaces of Superhydrophilic Polymer Brushes. Langmuir 2012, 28, 7212–7222. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Konno, T.; Takai, M.; Ishihara, K. Bioconjugated Phospholipid Polymer Biointerface for Enzyme-Linked Immunosorbent Assay. Biomacromolecules 2008, 9, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Takai, M.; Ishihara, K. Suppression of Protein Adsorption on a Charged Phospholipid Polymer Interface. Biomacromolecules 2009, 10, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Chang, Y.; Jiang, S. Surface Grafted Sulfobetaine Polymers via Atom Transfer Radical Polymerization as Superlow Fouling Coatings. J. Phys. Chem. B 2006, 110, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chang, W.; Shih, Y.; Wei, T.; Hsiue, G. Zwitterionic Sulfobetaine-Grafted Poly(vinylidene fluoride) Membrane with Highly Effective Blood Compatibility via Atmospheric Plasma-Induced Surface Copolymerization. ACS Appl. Mater. Interfaces 2011, 3, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liao, S.; Higuchi, A.; Ruaan, R.; Chu, C.; Chen, W. A Highly Stable Nonbiofouling Surface with Well-Packed Grafted Zwitterionic Polysulfobetaine for Plasma Protein Repulsion. Langmuir 2008, 24, 5453–5458. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.; Wang, M.; Chien, H.; Wei, T.; Lee, C.; Tsai, W. Surface Modification with Poly(sulfobetaine methacrylate-co-acrylic acid) To Reduce Fibrinogen Adsorption, Platelet Adhesion, and Plasma Coagulation. Biomacromolecules 2011, 12, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Jiang, S. Dual-Functional Biomimetic Materials: Nonfouling Poly(carboxybetaine) with Active Functional Groups for Protein Immobilization. Biomacromolecules 2006, 7, 3311–3315. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Pursuing “Zero” Protein Adsorption of Poly(carboxybetaine) from Undiluted Blood Serum and Plasma. Langmuir 2009, 25, 11911–11916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, B.; Luo, S.-C.; Lin, H.-A.; Nakao, A.; Yamashita, Y.; Yu, H.-H. Controlled Protein Absorption and Cell Adhesion on Polymer-Brush-Grafted Poly(3,4-ethylenedioxythiophene) Films. ACS Appl. Mater. Interfaces 2013, 5, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, J.; Wang, K.; Zhu, J. Highly Sensitive Mechanochromic Photonic Hydrogels with Fast Reversibility and Mechanical Stability. Langmuir 2015, 31, 8732–8737. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, Y.; Zhao, J.; Wang, Z.; Gao, M.; Gianneschi, N.C.; Dhinojwala, A.; Shawkey, M.D. Stimuli-Responsive Structurally Colored Films from Bioinspired Synthetic Melanin Nanoparticles. Chem. Mater. 2016, 28, 5516–5521. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, T.; Hu, L.; Gong, H.; Chen, C.; Chen, X.; Cai, C. Development of a Thermosensitive Molecularly Imprinted Polymer Resonance Light Scattering Sensor for Rapid and Highly Selective Detection of Hepatitis A Virus in Vitro. Sens. Actuators B 2017, 253, 1188–1193. [Google Scholar] [CrossRef]
- Lee, K.M.; Oh, Y.; Chang, J.Y.; Kim, H. Facile Fluorescent Labeling of a Polyacrylamide-Based Hydrogel Film via Radical Initiation Enables Selective and Reversible Detection of Al3+. J. Mater. Chem. B 2018, 6, 1244–1250. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Butt, H.; Yun, S.-H. Photonic Crystal Flakes. ACS Sens. 2016, 1, 493–497. [Google Scholar] [CrossRef]
- Jia, X.; Wang, K.; Wang, J.; Hu, Y.; Shen, L.; Zhu, J. Full-Color Photonic Hydrogels for pH and Ionic Strength Sensing. Eur. Polym. J. 2016, 83, 60–66. [Google Scholar] [CrossRef]
- Lu, W.; Li, H.; Huo, B.; Meng, Z.; Xue, M.; Qiu, L.; Ma, S.; Yan, Z.; Piao, C.; Ma, X. Full-Color Mechanical Sensor Based on Elastic Nanocomposite Hydrogels Encapsulated Three-Dimensional Colloidal Arrays. Sens. Actuators B 2016, 234, 527–533. [Google Scholar] [CrossRef]
- Aliabadi, A.; Rounaghi, G.H.; Zavar, M.H.A. A New Droplet-Based Polymeric Banana Electrochemical Biosensor for Analysis of One Microliter Solution of Paracetamol. Sens. Actuators B 2017, 241, 182–189. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Berg, D.; Duan, J.; Mugo, S.M.; Serpe, M.J. Optical Devices Constructed from Ferrocene-Modified Microgels for H2O2 Sensing. ACS Appl. Mater. Interfaces 2016, 8, 27264–27269. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Wagner, B.; Karbarz, M.; Nowicka, A.M. A Dual DNA Biosensor Based on Two Redox Couples with a Hydrogel Sensing Platform Functionalized with Carboxyl Groups and Gold Nanoparticles. Sens. Actuators B 2015, 208, 220–227. [Google Scholar] [CrossRef]
- Lifson, M.A.; Carter, J.A.; Miller, B.L. Functionalized Polymer Microgel Particles Enable Customizable Production of Label-Free Sensor Arrays. Anal. Chem. 2015, 87, 7887–7893. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Cho, E.C. Dual-responsive and Multifunctional Plasmonic Hydrogel Valves and Biomimetic Architectures Formed with Hydrogel and Gold Nanocolloids. Sci. Rep. 2016, 6, 34622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-T.; Cai, Z.; Kwak, D.H.; Liu, X.; Asher, S.A. Two-Dimensional Photonic Crystal Sensors for Visual Detection of Lectin Concanavalin A. Anal. Chem. 2014, 86, 9036–9041. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.R.C.; Sheng, Y.; Callan, B.; Donnelly, R.F.; Callan, J.F. A Hydrogel Based Zinc(II) Sensor for Use in Fluorescent Multi-Well Plate Analysis. New J. Chem. 2015, 39, 3461–3466. [Google Scholar] [CrossRef]
- Gong, D.; Cao, T.; Han, S.-C.; Zhu, X.; Iqbal, A.; Liu, W.; Qin, W.; Guo, H. Fluorescence Enhancement Thermoresponsive Polymer Luminescent Sensors Based on BODIPY for Intracellular Temperature. Sens. Actuators B 2017, 252, 577–583. [Google Scholar] [CrossRef]
- Zhang, J.; Kruss, S.; Hilmer, A.J.; Shimizu, S.; Schmois, Z.; De La Cruz, F.; Barone, P.W.; Reuel, N.F.; Heller, D.A.; Strano, M.S. A Rapid, Direct, Quantitative, and Label-Free Detector of Cardiac Biomarker Troponin T Using Near-Infrared Fluorescent Single-Walled Carbon Nanotube Sensors. Adv. Healthcare Mater. 2014, 3, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Gomulya, W.; Costanzo, G.D.; de Carvalho, E.J.; Bisri, S.Z.; Derenskyi, V.; Fritsch, M.; Fröhlich, N.; Allard, S.; Gordiichuk, P.; Herrmann, A.; et al. Semiconducting Single-Walled Carbon Nanotubes on Demand by Polymer Wrapping. Adv. Mater. 2013, 25, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Bisri, S.Z.; Gao, J.; Derenskyi, V.; Gomulya, W.; Iezhokin, I.; Gordiichuk, P.; Herrmann, A.; Loi, M.A. High Performance Ambipolar Field-Effect Transistor of Random Network Carbon Nanotubes. Adv. Mater. 2012, 24, 6147–6152. [Google Scholar] [CrossRef] [PubMed]
- Derenskyi, V.; Gomulya, W.; Rios, J.M.; Fritsch, M.; Fröhlich, N.; Jung, S.; Allard, S.; Bisri, S.Z.; Gordiichuk, P.; Herrmann, A.; et al. Carbon Nanotube Network Ambipolar Field-Effect Transistors with 108 On/Off Ratio. Adv. Mater. 2014, 26, 5969–5975. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Mai, C.; Zhang, K. Formation of Uniform Multi-Stimuli-Responsive and Multiblock Hydrogels from Dialdehyde Cellulose. ACS Sustain. Chem. Eng. 2017, 5, 5313–5319. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Cao, W.-T.; Ma, M.-G.; Wan, P. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Selfhealing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Nishiyabu, R.; Ushikubo, S.; Kamiya, Y.; Kubo, Y. A Boronate Hydrogel Film Containing Organized Two-Component Dyes as a Multicolor Fluorescent Sensor for Heavy Metal Ions in Water. J. Mater. Chem. A 2014, 2, 15846–15852. [Google Scholar] [CrossRef]
- Thombre, S.M.; Sarwade, B.D. Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review. J. Macromol. Sci. A 2005, 42, 1299–1315. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, L.Y.; Wu, S.; Wu, D.; Qin, X.; Ko, F. A Reversible Colorimetric Chemosensor for Naked-Eye Detection of Copper Ions Using Poly(aspartic acid) Nanofibrous Hydrogel. Dyes Pigments 2015, 123, 380–385. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Jiang, X.; Yin, J. Versatile Functionalization of the Micropatterned Hydrogel of Hyperbranched Poly(ether amine) Based on “Thiol-yne” Chemistry. Adv. Funct. Mater. 2014, 24, 1679–1686. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Li, Y.; Zeng, L.; Zhu, J.; Wang, G.; Chen, X. Diels–Alder Crosslinked HA/PEG Hydrogels with High Elasticity and Fatigue Resistance for Cell Encapsulation and Articular Cartilage Tissue Repair. Polym. Chem. 2014, 5, 5116–5123. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and Applications of Interpenetrating Polymer Network Hydrogels. A Review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Si, Y.; Wang, L.; Wang, X.; Tang, N.; Yu, J.; Ding, B. Ultrahigh-Water-Content, Superelastic, and Shape-Memory Nanofiber-Assembled Hydrogels Exhibiting Pressure-Responsive Conductivity. Adv. Mater. 2017, 29, 1700339. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, Healable, and Adhesive Epidermal Sensors Assembled from Mussel-Inspired Conductive Hybrid Hydrogel Framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Mandal, D.; Mandal, S.K.; Ghosh, M.; Das, P.K. Phenylboronic Acid Appended Pyrene-Based Low-Molecular-Weight Injectable Hydrogel: Glucose-Stimulated Insulin Release. Chem. Eur. J. 2015, 21, 12042–12052. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, D.; Tang, N.; Wu, J. Tb3+-Containing Supramolecular Hydrogels: Luminescence Properties and Reversible Sol–Gel Transitions Induced by External Stimuli. Dalton Trans. 2014, 43, 9856–9859. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, C.; Zhang, L.; Liu, M. Visualized Discrimination of ATP from ADP and AMP through Collapse of Supramolecular Gels. Chem. Commun. 2014, 50, 12688–12690. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Cheng, Q.; Zhang, Q.; Wang, Y.; Chang, C.; Li, L.; Peng, H.; Zhang, L. Deformation Drives Alignment of Nanofibers in Framework for Inducing Anisotropic Cellulose Hydrogels with High Toughness. ACS Appl. Mater. Interfaces 2017, 9, 43154–43162. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, J.Y. Reversible Thermochromic Polymer Film Embedded with Fluorescent Organogel Nanofibers. Langmuir 2014, 30, 13673–13679. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ryu, J.H.; Kim, H.K.; Chang, J.Y. A Versatile Platform for Lanthanide(III)-Containing Organogelators: Fabrication of the Er(III)-Incorporated Polymer Nanocomposite from an Organogel Template. New J. Chem. 2017, 41, 12366–12370. [Google Scholar] [CrossRef]
- Lee, C.; Ko, Y.-J.; Lee, S.-Y. A Pyrocatechol Violet-Titanium Alkoxide Complex for HF Sensing: Study on the Complex Structure and Application. Dyes Pigments 2016, 127, 133–141. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired Mineral Hydrogel as a Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Chang, J.Y. Polymers for Luminescent Sensing Applications. Macromol. Chem. Phys. 2014, 215, 1274–1285. [Google Scholar] [CrossRef]
- Kim, H.; Cha, M.C.; Park, H.W.; Chang, J.Y. Preparation of a Yb(III)-Incorporated Porous Polymer by Post-Coordination: Enhancement of Gas Adsorption and Catalytic Activity. J. Polym. Sci. A 2013, 51, 5291–5297. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, J.Y. Fabrication of a Fluorescent Sensor by Organogelation: CdSe/ZnS Quantum Dots Embedded Molecularly Imprinted Organogel Nanofibers. Sens. Actuators B 2016, 234, 122–129. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.M.; Chang, J.Y. Highly Luminescent Tetra(biphenyl-4-yl)ethene-grafted Molecularly Imprinted Mesoporous Silica Nanoparticles for Fluorescent Sensing of Diethylstilbestrol. Sens. Actuators B 2017, 242, 1296–1304. [Google Scholar] [CrossRef]
- EL-Sharif, H.F.; Aizawa, H.; Reddy, S.M. Spectroscopic and Quartz Crystal Microbalance (QCM) Characterisation of Protein-Based MIPs. Sens. Actuators B 2015, 206, 239–245. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, H.J.; Jung, D.; Oh, Y.; Lee, H.; Han, C.; Chang, J.Y.; Kim, H. Rapid Accessible Fabrication and Engineering of Bilayered Hydrogels: Revisiting the Cross-Linking Effect on Superabsorbent Poly(acrylic acid). ACS Omega 2018, 3, 3096–3103. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Naicker, S.; Kaur, K.; Thundat, T. Metabolic Study of Cancer Cells Using a pH Sensitive Hydrogel Nanofiber Light Addressable Potentiometric Sensor. ACS Sens. 2017, 2, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Rodrigues, M.-T.F.; Song, Z.; Li, H.; Xu, H.; Liu, H.; Wu, J.; Xu, Y.; Song, Y.; Liu, Y.; et al. Reversible Formation of g-C3N4 3D Hydrogels through Ionic Liquid Activation: Gelation Behavior and Room-Temperature Gas-Sensing Properties. Adv. Funct. Mater. 2017, 27, 1700653. [Google Scholar] [CrossRef]
- Seo, S.; Lee, J.; Kwon, M.S.; Seo, D.; Kim, J. Stimuli-Responsive Matrix-Assisted Colorimetric Water Indicator of Polydiacetylene Nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 20342–20348. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon Dots Rooted Agarose Hydrogel Hybrid Platform for Optical Detection and Separation of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2015, 7, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Mao, Z.; Qing, T.; He, X.; Zou, Z.; He, D.; Shi, H.; Huang, J.; Liu, J.; Wang, K. Visual and Portable Strategy for Copper(II) Detection Based on a Striplike Poly(Thymine)-Caged and Microwell-Printed Hydrogel. Anal. Chem. 2014, 86, 11263–11268. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.T.; Chung, J.S.; Hur, S.H. A Highly Sensitive Enzyme-Free Glucose Sensor Based on Co3O4 Nanoflowers and 3D Graphene Oxide Hydrogel Fabricated via Hydrothermal Synthesis. Sens. Actuators B 2016, 223, 76–82. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, A.; Zhao, M.; Dong, W.; Zhao, T.; Wang, J.; Tang, W. Bimetallic PdCu Nanoparticle Decorated Three-dimensional Graphene Hydrogel for Non-Enzymatic Amperometric Glucose Sensor. Sens. Actuators B 2014, 190, 707–714. [Google Scholar] [CrossRef]
- Kim, H.; Mohapatra, H.; Phillips, S.T. Rapid, On-Command Debonding of Stimuli-Responsive Cross-Linked Adhesives by Continuous, Sequential Quinone Methide Elimination Reactions. Angew. Chem. Int. Ed. 2015, 54, 13063–13067. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.S.; Kim, H.; Olah, M.G.; Lewis, G.G.; Phillips, S.T. Depolymerizable Poly(benzyl ether)-Based Materials for Selective Room Temperature Recycling. Green Chem. 2015, 17, 4541–4545. [Google Scholar] [CrossRef]
- Yeung, K.; Kim, H.; Mohapatra, H.; Phillips, S.T. Surface-Accessible Detection Units in Self-Immolative Polymers Enable Translation of Selective Molecular Detection Events into Amplified Responses in Macroscopic, Solid-State Plastics. J. Am. Chem. Soc. 2015, 137, 5324–5327. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, H.; Kim, H.; Phillips, S.T. Stimuli-Responsive Polymer Film that Autonomously Translates a Molecular Detection Event into a Macroscopic Change in Its Optical Properties via a Continuous, Thiol-Mediated Self-Propagating Reaction. J. Am. Chem. Soc. 2015, 137, 12498–12501. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Baker, M.S.; Phillips, S.T. Polymeric Materials that Convert Local Fleeting Signals into Global Macroscopic Responses. Chem. Sci. 2015, 6, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
























| Classification | Reaction scheme | Refs. |
|---|---|---|
| Thiol-ene addition: The anti-Markovnikov addition of thiols to alkenes is facilitated by a radical source or by UV irradiation. |  | [45,46] |
| Thiol-disulfide exchange: This type of reaction is frequently found in biological systems. Disulfides as pyridyl disulfide are readily exchanged in high yields with thiol compounds. |  | [47,48] |
| Epoxides, anhydrides, isocyanates: These are a class of reactive groups, that are, importantly, tolerant toward radical-based polymerization methods. |  | [49,50,51] |
| Ketones and aldehydes: These can selectively react with primary amines, alkoxyamines, and hydrazines, producing imines, oximes, and hydrozones, respectively. |  | [52,53] |
| Active esters: The reaction of active ester groups with amines can proceeds selectively even in the presence of weaker nucleophiles, such as alcohols. |  | [54,55,56] |
| Diels–Alder cycloaddition: A diene and a substituted alkene can make cycloaddition reaction, which is reversible. |  | [57,58] |
| Michael addition: Thiols undergo Michael-type addition to activated alkenes, which proceeds rapidly in aqueous media under mild conditions. |  | [59,60,61] |
| Type of Reaction | Functional Group or Materials | Refs. | |
|---|---|---|---|
| Covalent Chemistry | Radical polymerization | Vinyl monomers | [85,86,87,88,89,90,98,99] |
| Radical copolymerization | Vinyl monomers | [91,92,93,94,101] | |
| Esterification 1 | Carboxylic acid–alcohol | [95] | |
| Amidation 1 | Carboxylic acid–amine | [96,97,106,110] | |
| Imine condensation 1 | Imine–aldehydes | [102,106] | |
| Ketal formation 1 | Diol–ketone | [107] | |
| Boron esterification | Boronic acid–diol | [98] | |
| Thiol-yne | Thiol–terminal alkyne | [111] | |
| Diels–Alder | Furan–maleimide | [112] | |
| Non-covalent Chemistry | Metal coordination | Carboxylic acid–metal | [114,133,134,135] |
| Sodium borate–diol | [115] | ||
| Self-assembly/crystallization | Small molecule | [116,117,118] | |
| Polymers | [119] | ||
| Miscellaneous | Additive processing | Synthetic dye | [123] |
| CaCO3 crystal | [124] | ||
| Molecular imprinting | Acrylamides | [129] | |
| Multilayered structure | PAA | [130] | |
| Electrospinning | PAA | [131] | |
| Hydrothermal method | Graphene | [132,136,137] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.M.; Kim, K.H.; Yoon, H.; Kim, H. Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale. Polymers 2018, 10, 551. https://doi.org/10.3390/polym10050551
Lee KM, Kim KH, Yoon H, Kim H. Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale. Polymers. 2018; 10(5):551. https://doi.org/10.3390/polym10050551
Chicago/Turabian StyleLee, Kyoung Min, Kyung Ho Kim, Hyeonseok Yoon, and Hyungwoo Kim. 2018. "Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale" Polymers 10, no. 5: 551. https://doi.org/10.3390/polym10050551
APA StyleLee, K. M., Kim, K. H., Yoon, H., & Kim, H. (2018). Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale. Polymers, 10(5), 551. https://doi.org/10.3390/polym10050551