On the Potential of Using Dual-Function Hydrogels for Brackish Water Desalination
Abstract
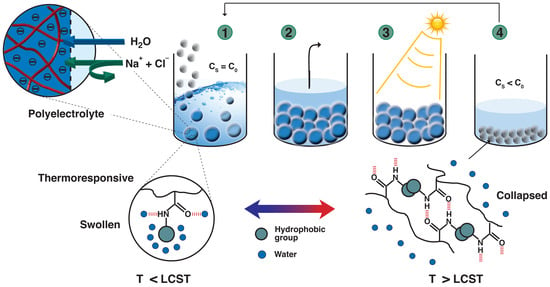
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogels
2.2.1. Synthesis of the Poly(acrylic acid)macromolecular RAFT Agents (PAAc-TTC)
2.2.2. Synthesis of Comb-Type Grafted PNIPAAm-g-PAAc Networks
2.3. Characterisation and Measurements
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Swelling Ratio and Kinetics of Swelling
2.3.6. Determination of Water Recovery and Salt Rejection
2.3.7. Rheological Measurement
2.3.8. Mesh Size Calculation of Hydrogels
3. Results and Discussion
3.1. Synthesis of Hydrogels
3.2. Characterisation of Hydrogels
3.2.1. Swelling Properties
3.2.2. Rheological Investigation and Network Structure
3.3. Dewatering Behaviour of Hydrogels and Salt Rejection
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Buchholz, F.L.; Graham, A.T. (Eds.) Modern Superabsorbent Polymer Technology, 1st ed.; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Dusek, K. (Ed.) Responsive Gels: Volume Transitions 1 (Advances in Polymer Science), 1st ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 109. [Google Scholar]
- Yang, Q.; Adrus, N.; Tomicki, F.; Ulbricht, M. Composites of functional polymeric hydrogels and porous membranes. J. Mater. Chem. 2011, 21, 2783–2811. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Kosik, K.; Wilk, E.; Geissler, E.; László, K. Influence of a Crown Ether Comonomer on the Temperature- Induced Phase Transition of Poly(N-isopropylacrylamide) Hydrogels. J. Phys. Chem. B 2008, 112, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry, 1st ed.; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Höpfner, J.; Klein, C.; Wilhelm, M. A Novel Approach for the Desalination of Seawater by Means of Reusable Poly(acrylic acid) Hydrogels and Mechanical Force. Macromol. Rapid Commun. 2010, 31, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Höpfner, J.; Richter, T.; Košovan, P.; Holm, C.; Wilhelm, M. Seawater Desalination via Hydrogels: Practical Realisation and First Coarse Grained Simulations. In Intelligent Hydrogels; Sadowski, G., Richtering, W., Eds.; Progress in Colloid and Polymer Science; Springer International Publishing: Cham, Switzerland, 2013; Volume 140, pp. 247–263. [Google Scholar]
- Schild, G.H. Poly(N-isopropylacrylamide): Experiment, Theory, and Application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Flory, J.P.; Rehner, J. Statistical Mechanism of Cross-linked Polymer Networks. I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.T. Smart draw agents for emerging forward osmosis application. J. Mater. Chem. A 2013, 45, 14049–14060. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, R.; Krantz, W.B.; Fane, A.G.; Hu, X.M. Exploration of using thermally responsive polyionic liquid hydrogels as draw agents in forward osmosis. RSC Adv. 2015, 118, 97143–97150. [Google Scholar] [CrossRef]
- Fänger, C.; Wack, H.; Ulbricht, M. Macroporous Poly(N-isopropylacrylamide) Hydrogels with Adjustable Size Cut-off for the Efficient and Reversible Immobilization of Biomacromolecules. Macromol. Biosci. 2006, 6, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Righetti, P.G.; Gelfi, C. Electrophoresis Gel Media: The State of the Art. J. Chromatogr. B 1997, 699, 63–75. [Google Scholar] [CrossRef]
- Marchetti, M.; Cussler, E.L. Hydrogels as Ultrafiltration Devices. Sep. Purif. Methods 1989, 18, 177–192. [Google Scholar] [CrossRef]
- Cussler, E.L.; Stokar, M.R.; Varberg, J.E. Gels as Size Selective Extraction Solvents. AlChE J. 1984, 30, 578–582. [Google Scholar] [CrossRef]
- Freitas, R.F.S.; Cussler, E.L. Temperature Sensitive Gels as Extraction Solvents. Chem. Eng. Sci. 1987, 42, 97–103. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: London, UK; San Diego, CA, USA, 2011. [Google Scholar]
- Ali, W.; Gebert, B.; Hennecke, T.; Graf, K.; Ulbricht, M.; Gutmann, J.S. Design of Thermally Responsive Polymeric Hydrogels for Brackish Water Desalination: Effect of Architecture on Swelling, Deswelling, and Salt Rejection. ACS Appl. Mater. Interfaces 2015, 7, 15696–15706. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.F.; Zhang, P.; Lu, M.G. Synthesis and swelling behavior of comb-type grafted hydrogels by reversible addition-fragmentation chain transfer polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2615–2624. [Google Scholar] [CrossRef]
- Liu, Q.F.; Zhang, P.; Qing, A.X.; Lan, Y.X.; Lu, M.G. Poly(N-isopropylacrylamide) hydrogels with improved shrinking kinetics by RAFT polymerization. Polymer 2006, 47, 2330–2336. [Google Scholar] [CrossRef]
- Velasquez, E.; Pembouong, G.; Rieger, J.; Stoffelbach, F.; Boyron, O.; Charleux, B.; D’Agosto, F.; Lansalot, M.; Dufils, P.E.; Vinas, J. Poly(vinylidene chloride)-Based Amphiphilic Block Copolymers. Macromolecules 2013, 46, 664–673. [Google Scholar] [CrossRef]
- Mansor, B.A.; Huglin, M.B. DSC studies on states of water in crosslinked poly(methyl methacrylate-co-n-vinyl-2-pyrrolidone) hydrogels. Polym. Int. 1994, 33, 273–277. [Google Scholar]
- Canal, T.; Peppas, N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ugaz, V.M. Using in situ rheology to characterize the microstructure in photopolymerized polyacrylamide gels for DNA electrophoresis. Electrophoresis 2006, 27, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Barner-Kowollik, C.; Quinn, J.F.; Vana, P.; Davis, T.P. Origin of Inhibition Effects in the Reversible Addition Fragmentation Chain Transfer (RAFT) Polymerization of Methyl Acrylate. Macromolecules 2002, 35, 8300–8306. [Google Scholar] [CrossRef]
- Chen, G.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Tabata, Y.; Ikada, Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials 1999, 20, 1339–1344. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Yang, Y.Y.; Chung, T.S. The Influence of Cold Treatment on Properties of Temperature-Sensitive Poly(N-isopropylacrylamide) Hydrogels. J. Colloid Interface Sci. 2002, 246, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug. Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Donnan, F.G.; Guggenheim, E.A. Exact thermodynamics of membrane equilibrium. Z. Phys. Chem. 1932, A162, 346–360. [Google Scholar]



















| Expt. | Macro-RAFT Agent | (mol L) | Time (min) | Conv. (mol %) | (g mol) | (g mol) | |
|---|---|---|---|---|---|---|---|
| E 1 | PAAc3K | 1.50 | 90 | 80 | 45.5 | 3320 | 3560 |
| E 2 | PAAc5K | 2.07 | 130 | 80 | 48.8 | 4930 | 5071 |
| E 3 | PAAc10K | 3.33 | 233 | 80 | 56.4 | 9830 | 10551 |
| Expt. | Gel Code | PAAc-TTC (wt %) | NIPAAm (wt %) | NIPAAm Conv. (wt %) | PAAc-TTC Content (wt %) |
|---|---|---|---|---|---|
| G1 | GG3K–20 | 20 | 80 | 98.4 | 20.3 |
| G2 | GG3K–30 | 30 | 70 | 96.2 | 31.1 |
| G3 | GG3K–40 | 40 | 60 | 94.7 | 42.2 |
| G4 | GG3K–50 | 50 | 50 | – | – |
| G5 | GG5K–20 | 20 | 80 | 98.0 | 20.4 |
| G6 | GG5K–30 | 30 | 70 | 97.2 | 30.8 |
| G7 | GG5K–40 | 40 | 60 | 93.5 | 42.6 |
| G8 | GG5K–50 | 50 | 50 | – | – |
| G9 | GG10K–20 | 20 | 80 | 96.8 | 20.6 |
| G10 | GG10K–30 | 30 | 70 | 95.1 | 31.5 |
| G11 | GG10K–40 | 40 | 60 | 93.4 | 42.6 |
| G12 | GG10K–50 | 50 | 50 | – | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, W.; Gebert, B.; Altinpinar, S.; Mayer-Gall, T.; Ulbricht, M.; Gutmann, J.S.; Graf, K. On the Potential of Using Dual-Function Hydrogels for Brackish Water Desalination. Polymers 2018, 10, 567. https://doi.org/10.3390/polym10060567
Ali W, Gebert B, Altinpinar S, Mayer-Gall T, Ulbricht M, Gutmann JS, Graf K. On the Potential of Using Dual-Function Hydrogels for Brackish Water Desalination. Polymers. 2018; 10(6):567. https://doi.org/10.3390/polym10060567
Chicago/Turabian StyleAli, Wael, Beate Gebert, Sedakat Altinpinar, Thomas Mayer-Gall, Mathias Ulbricht, Jochen S. Gutmann, and Karlheinz Graf. 2018. "On the Potential of Using Dual-Function Hydrogels for Brackish Water Desalination" Polymers 10, no. 6: 567. https://doi.org/10.3390/polym10060567
APA StyleAli, W., Gebert, B., Altinpinar, S., Mayer-Gall, T., Ulbricht, M., Gutmann, J. S., & Graf, K. (2018). On the Potential of Using Dual-Function Hydrogels for Brackish Water Desalination. Polymers, 10(6), 567. https://doi.org/10.3390/polym10060567