Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
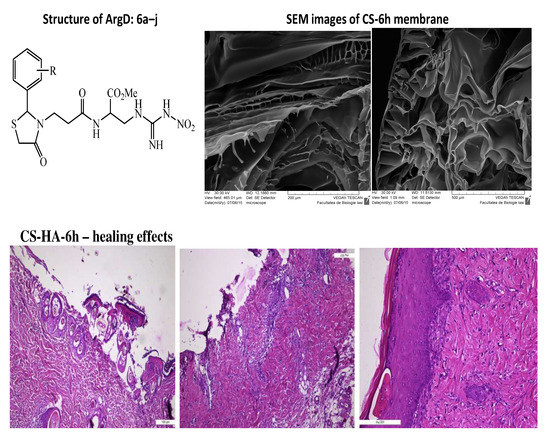
2.2. Synthesis of Arginine Derivatives
2.3. Preparation of Chitosan—Arginine Derivatives (CS-ArgD) Membranes
2.4. Preparation of Chitosan-Hyaluronic Acid-Arginine Derivatives (CS-HA-ArgD) Membranes
2.5. Characterization of Chitosan/Chitosan-Hyaluronic Acid-Arginine Derivatives (CS-ArgD, CS-HA-ArgD) Membranes
2.5.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.5.2. Morphology
2.5.3. Porosity Test
2.5.4. Swelling Ratio
2.5.5. Contact Angle Measurements
2.5.6. Surface Tension Parameters
2.6. Biological Evaluation
2.6.1. Wound Healing Assay
2.6.2. Statistical Analysis
3. Results
3.1. Chemistry
3.2. Characterization of Chitosan/Chitosan-Hyaluronic Acid-Arginine Derivatives (CS-ArgD, CS-HA-ArgD) Membranes
3.2.1. FT-IR Spectral Data
3.2.2. Morphology
3.2.3. Porosity Test
3.2.4. Swelling Degree
3.2.5. Surface Tension Parameters
3.3. Biological Evaluation
Wound Healing Assay
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, R.; Nakamura, M.; Okabayashi, T.; Tanaka, Y.; Nagai, A.; Yamashita, K. Efficacy of polarized hydroxyapatite and silk fibroin composite dressing gel on epidermal recovery from full-thickness skin wounds. J. Biomed. Mater. Res. 2009, 90, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Mirzaei, S.; Tang, Y. Cost-effective double-layer hydrogel composites for wound dressing applications. Polymers 2018, 10, 305. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2017, 35, 489–494. [Google Scholar]
- Pintilie, L.; Negut, C.; Oniscu, C.; Caproiu, M.T.; Nechifor, M.; Iancu, L.; Ghiciuc, C.; Ursu, R. Synthesis and antibacterial activityof some novel quinolones. Rom. Biotechnol. Lett. 2009, 14, 4756–4767. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wan, Y.Y.; Zhao, M.; Liu, Y.; Zhang, S. Preparation and characterization of antimicrobial chitosan-N-arginine with different degrees of substitution. Carbohydr. Polym. 2011, 83, 144–150. [Google Scholar] [CrossRef]
- Ye, M.; Lian, X.; Huaping, T.; Ming, F.; Jianliang, L.; Yang, J.; Zhonghua, L.; Yong, C.; Xiaohong, H. Chitosan membrane dressings toughened by glycerol to load antibacterial drugs for wound healing. Mater. Sci. Eng. 2017, 81, 522–531. [Google Scholar]
- Nitta, S.; Kaketani, S.; Iwamoto, H. Development of chitosan-nanofiber-based hydrogels exhibiting high mechanical strength and pH-responsive controlled release. Eur. Polym. J. 2015, 67, 50–56. [Google Scholar] [CrossRef]
- Castro, S.P.M.; Lizárraga Paulín, E.G. Chitosan a new panacea? Areas of application. In The Complex World of Polysaccharides; Intech: Luxembourg, 2012; pp. 3–46. [Google Scholar]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Lupascu, F.G.; Dash, M.; Samal, S.K.; Dubruel, P.; Lupușoru, C.E.; Lupusoru, R.-V.; Dragostin, O.; Profire, L. Development, optimization and biological evaluation of chitosan scaffold formulations of new xanthine derivatives for treatment of type-2 diabetes mellitus. Eur. J. Pharm. Sci. 2015, 77, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Reys, L.L.; Silva, S.S.; Pirraco, R.P.; Marques, A.P.; Mano, J.F.; Silva, T.H.; Reis, R.L. Influence of freezing temperature and deacetylation degree on the performance of freeze-dried chitosan scaffolds towards cartilage tissue engineering. Eur. Polym. J. 2017, 95, 232–240. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, B.J.; Weng, Y.M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Mercy, H.P.; Halim, A.S.; Hussein, A.R. Chitosan-derivatives as hemostatic agents: Their role in tissue regeneration. Neural Regen. Res. 2012, 1, 38–46. [Google Scholar]
- Lee, J.; Duncan, A.; Townsend, S.; Baker, S. Synthesis and characterization of a chitosan derivative 976.3. FASEB J. 2014, 28, 1–9. [Google Scholar]
- Wang, X.; Yan, Y.; Zhang, R. A comparison of chitosan and collagen sponges as hemostatic dressings. J. Bioact. Compat. Polym. 2006, 21, 39–54. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchiluș, C.; Ghețu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Carréra Silva, J.O., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A comprehensive review of advanced biopolymericwoundhealing systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Campisi, M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers forwoundsand burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Basset Sanada, R.; Abdel-Bar, H.M. Chitosan-hyaluronic acid composite sponge scaffold enriched with Andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr. Polym. 2017, 173, 441–450. [Google Scholar]
- Mohandas, A.; Anisha, B.S.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Colloids Surf. B Biointerfaces 2015, 127, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, E.; Ossipov, D.A.; Hilborn, J.; Larsson, S.; Jonsson, K.B.; Varghese, O.P. Bone reservoir: Injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J. Control. Release 2011, 152, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, G.; Kim, M.R.; Mohammed, S.I.; Yeo, Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J. Control. Release 2012, 158, 86–392. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Ning, W.; Xun, J.; Deng, R.; Shihong, N.; Sun, L.; Qinjie, W.; Yuquan, W.; Changyang, G. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar]
- Schantéa, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Iwasaki, N.; Kasahara, Y.; Yamane, S.; Igarashi, T.; Minami, A.; Nisimura, S. Chitosan-based hyaluronic acid hybrid polymer fibers as a scaffold biomaterial for cartilage tissue engineering. Polymers 2011, 3, 100–113. [Google Scholar] [CrossRef]
- Gould, A.; Naidoo, C.; Candy, G.P. Arginine metabolism and wound healing. Wound Heal. S. Afr. 2008, 1, 48–50. [Google Scholar]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debats, I.B.J.G.; Wolfs, T.G.A.M.; Gotoh, T.; Cleutjens, J.P.M.; Peutz-Kootstra, C.J.; van der Hulst, R.R.W.J. Role of arginine in superficial wound healing in man. Nitric Oxide 2009, 21, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Pânzariu, A.T.; Apotrosoaei, M.; Vasincu, I.M.; Drăgan, M.; Constantin, S.; Buron, F.; Routier, S.; Profire, L.; Tuchiluș, C. Synthesis and biological evaluation of new 1,3-thiazolidine-4-one derivatives of nitro-L-arginine nethyl ester. Chem. Cent. J. 2016, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Anisha, B.S.; Sankar, D.; Mohandas, A.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Chitosan–hyaluronan/nano chondroitin sulfate ternary composite sponges for medical use. Carbohydr. Polym. 2013, 92, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Lua, B.; Lua, F.; Zou, Y.; Jiawei, L.; Rong, B.; Zhiquan, L.; Fangying, D.; Dayang, W.; Guangqian, L. In situ reduction of silver nanoparticles by chitosan-l-glutamic acid/hyaluronic acid: Enhancing antimicrobial and wound-healing activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Tan, F.; Marra, K.G.; Jan, S.S.; Liu, D.C. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009, 5, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Rotta, J.; Ozório, R.A.; Kehrwald, A.M.; Mariz de Oliveira Barra, G.; Dias de Melo Castanho Amboni, R.; Barreto, P.L.M. Parameters of color, transparency, water solubility, wettability and surface free energy of chitosan/hydroxypropyl-methylcellulose (HPMC) films plasticized with sorbitol. Mater. Sci. Eng. 2009, 29, 619–623. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchiluș, C.; Vasile, C.; Profire, L. Development and Characterization of Novel Films Based on Sulfonamide-Chitosan Derivatives for Potential Wound Dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. The Mechanical and biological properties of chitosan scaffolds for tissue regeneration templates are significantly enhanced by chitosan from Gongronella butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Zangi, S.; Hejazi, I.; Seyfi, J.; Hejazi, E.; Khonakdar, H.A.; Davachi, S.M. Tuning cell adhesion on polymeric and nanocomposite surfaces: Role of topography versus superhydrophobicity. Mater. Sci. Eng.C 2016, 63, 609–615. [Google Scholar] [CrossRef] [PubMed]











| CS-ArgD/CS-HA-ArgD | Contact Angle Value (°) | |||||
|---|---|---|---|---|---|---|
| Double-Distilled Water | Formamide | Diiodomethane | ||||
| CS | CS-HA | CS | CS-HA | CS | CS-HA | |
| CS/CS-HA | 102.14 ± 2.3 | 10.97 ± 1.9 | 69.86 ± 4.5 | 40.23 ± 2.2 | 65.16 ± 0.2 | 89.98 ± 1.1 |
| NO2-Arg-OMe | 102.52 ± 3.1 | 26.76 ± 2.3 | 47.77 ± 0.7 | 39.8 ± 1.9 | 54.00 ± 4.7 | 88.97 ± 0.5 |
| 6a | 50.88 ± 0.9 | 16.07 ± 0.5 | 47.79 ± 1.5 | 12.34 ± 2.6 | 41.99 ± 1.0 | 40.99 ± 3.8 |
| 6b | 79.07 ± 1.7 | 35.34 ± 4.6 | 56.44 ± 2.3 | 30.27 ± 3.5 | 47.68 ± 0.8 | 67.54 ± 2.9 |
| 6c | 77.17 ± 4.3 | 16.07 ± 0.5 | 51.83 ± 3.5 | 35.41 ± 0.7 | 46.81 ± 2.7 | 58.66 ± 1.7 |
| 6d | 57.84 ± 1.5 | 30.39 ± 2.1 | 39.22 ± 0.6 | 34.09 ± 4.3 | 43.77 ± 3.1 | 66.43 ± 1.6 |
| 6e | 74.88 ± 1.4 | 26.74 ± 3.0 | 59.50 ± 2.8 | 23.29 ± 0.8 | 46.87 ± 4.3 | 70.89 ± 2.3 |
| 6f | 71.35 ± 0.3 | 36.21 ± 2.9 | 55.93 ± 2.0 | 46.56 ± 1.6 | 39.86 ± 4.4 | 56.78 ± 3.3 |
| 6g | 73.15 ± 2.6 | 32.17 ± 0.3 | 59.79 ± 4.3 | 24.98 ± 1.8 | 44.45 ± 1.7 | 68.79 ± 0.7 |
| 6h | 85.90 ± 3.5 | 47.67 ± 1.9 | 62.21 ± 1.7 | 38.05 ± 4.1 | 53.93 ± 2.3 | 57.09 ± 4.4 |
| 6i | 83.36 ± 1.2 | 40.96 ± 1.1 | 61.89 ± 0.3 | 26.37 ± 2.9 | 37.21 ± 0.8 | 60.54 ± 4.6 |
| 6j | 84.98 ± 2.9 | 38.79 ± 3.8 | 54.78 ± 1.2 | 17.65 ± 2.5 | 51.18 ± 4.5 | 45.57 ± 2.4 |
| Films | The Surface Tension Parameters (mN/m) | |||||
|---|---|---|---|---|---|---|
| CS-ArgDfilms | ||||||
| CS | 25.56 | 1.52 | 0.04 | 0.52 | 26.08 | 31.31 |
| CS-PArg | 31.95 | 6.93 | 4.90 | 11.65 | 43.60 | 32.27 |
| CS-6a | 38.51 | 0.02 | 34.80 | 1.59 | 40.11 | 31.09 |
| CS-6b | 35.48 | 0.45 | 5.79 | 3.23 | 38.72 | 24.88 |
| CS-6c | 35.96 | 0.91 | 5.39 | 4.43 | 40.39 | 22.59 |
| CS-6d | 37.59 | 1.13 | 18.73 | 9.19 | 42.77 | 16.86 |
| CS-6e | 35.93 | 0.35 | 6.00 | 2.04 | 37.96 | 28.37 |
| CS-6f | 39.60 | 0.22 | 5.08 | 1.88 | 41.48 | 30.56 |
| CS-6g | 37.23 | 0.18 | 5.56 | 1.60 | 38.83 | 30.47 |
| CS-6h | 31.99 | 0.36 | 5.19 | 2.74 | 34.73 | 24.63 |
| CS-6i | 40.90 | 0.01 | 5.02 | 0.45 | 41.35 | 38.25 |
| CS-6j | 33.54 | 1.48 | 6.62 | 3.10 | 36.65 | 24.36 |
| CS-HA-ArgDfilms | ||||||
| CS-HA | 12.72 | 6.68 | 70.81 | 43.49 | 56.21 | 0.35 |
| CS-HA-NO2-Arg-OMe | 13.13 | 8.71 | 44.74 | 39.49 | 52.62 | 0.13 |
| CS-HA-6a | 39.03 | 1.42 | 51.58 | 17.13 | 56.16 | 12.06 |
| CS-HA-6b | 24.21 | 4.79 | 40.78 | 27.96 | 52.17 | 3.05 |
| CS-HA-6c | 29.29 | 2.73 | 31.51 | 18.55 | 47.83 | 7.30 |
| CS-HA-6d | 24.84 | 4.11 | 36.92 | 24.62 | 49.46 | 3.86 |
| CS-HA-6e | 22.33 | 6.43 | 45.61 | 34.26 | 56.59 | 1.94 |
| CS-HA-6f | 30.36 | 7.08 | 92.86 | 31.14 | 91.50 | 7.81 |
| CS-HA-6g | 23.50 | 5.96 | 41.07 | 31.29 | 54.79 | 2.46 |
| CS-HA-6h | 30.19 | 1.42 | 44.30 | 15.88 | 46.06 | 8.87 |
| CS-HA-6i | 28.21 | 3.56 | 43.63 | 24.93 | 53.13 | 4.97 |
| CS-HA-6j | 36.63 | 2.07 | 42.33 | 18.71 | 55.34 | 10.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacob, A.-T.; Drăgan, M.; Ghețu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers 2018, 10, 607. https://doi.org/10.3390/polym10060607
Iacob A-T, Drăgan M, Ghețu N, Pieptu D, Vasile C, Buron F, Routier S, Giusca SE, Caruntu I-D, Profire L. Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers. 2018; 10(6):607. https://doi.org/10.3390/polym10060607
Chicago/Turabian StyleIacob, Andreea-Teodora, Maria Drăgan, Nicolae Ghețu, Dragoș Pieptu, Cornelia Vasile, Frédéric Buron, Sylvain Routier, Simona Elena Giusca, Irina-Draga Caruntu, and Lenuța Profire. 2018. "Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives" Polymers 10, no. 6: 607. https://doi.org/10.3390/polym10060607
APA StyleIacob, A. -T., Drăgan, M., Ghețu, N., Pieptu, D., Vasile, C., Buron, F., Routier, S., Giusca, S. E., Caruntu, I. -D., & Profire, L. (2018). Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers, 10(6), 607. https://doi.org/10.3390/polym10060607