Critical Point Drying: An Effective Drying Method for Direct Measurement of the Surface Area of a Pretreated Cellulosic Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatments of Lignocellulosic Biomass
2.3. Drying of Cellulosic Biomass Sample
2.4. Surface Area and Pore Size Measurements of Biomass Sample
2.5. FTIR and SEM Analyses
3. Results and Discussion
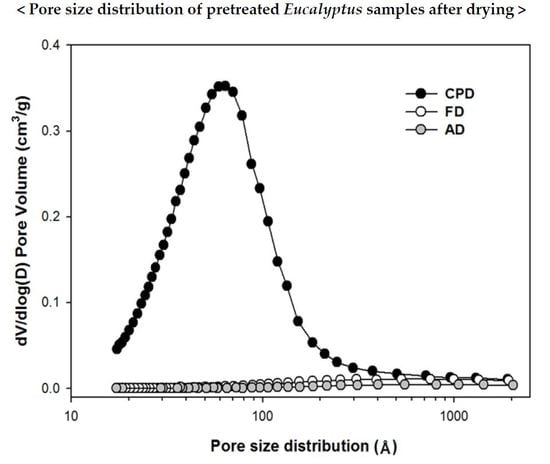
3.1. Drying Methods Effects on Surface Area and Pore Size Distribution of Pretreated Eucalyptus
3.2. Pretreatment Conditions Effects on Surface Area and Pore Size Distribution
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part 1: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Meng, X.; Ragauskas, A.J. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Grethlein, H.E. The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates. Nat. Biotechnol. 1985, 3, 155–160. [Google Scholar] [CrossRef]
- Stone, J.E.; Scallan, A.M. A structural model of the cell wall of water-swollen wood pulp fibers based on their accessibility to macromolecules. Cellul. Chem. Technol. 1968, 2, 321–342. [Google Scholar]
- Gama, F.M.; Teixeira, J.A.; Mota, M. Cellulose morphology and enzymatic reactivity: A modified solute exclusion technique. Biotechnol. Bioeng. 1994, 43, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Su, R.; Qi, W.; He, Z. Understanding the key factors for enzymatic conversion of pretreated lignocellulose by partial least square analysis. Biotechnol. Prog. 2010, 26, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, P.; Zhang, M.; Hu, J.; Xing, F. The pore structure of phosphoaluminate cement. J. Compos. Mater. 2012, 2, 104–112. [Google Scholar] [CrossRef]
- Hong, J.; Ye, X.; Zhang, Y.H. Quantitative determination of cellulose accessibility to cellulase based on adsorption of a nonhydrolytic fusion protein containing CBM and GFP with its applications. Langmuir 2007, 23, 12535–12540. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.F.; Chandra, R.P.; Saddler, J.N. The effects of increasing swelling and anionic charges on the enzymatic hydrolysis of organosolv–pretreated softwoods at low enzyme loadings. Biotechnol. Bioeng. 2011, 108, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Wiman, M.; Dienes, D.; Hansen, M.A.T.; van der Meulen, T.; Zacchi, G.; Lidén, G. Cellulose accessibility determines the rate of enzymatic hydrolysis of steam–pretreated spruce. Bioresour. Technol. 2012, 126, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhu, J.Y. Effects of drying–induced fiber hornification on enzymatic saccharification of lignocelluloses. Enzyme Microb. Technol. 2011, 48, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Foston, M.; Ragauskas, A.J. Changes in the structure of the cellulose fiber wall during dilute acid pretreatment in Populus studied by 1H and 2H NMR. Energy Fuels 2010, 24, 5677–5685. [Google Scholar] [CrossRef]
- Meng, X.; Foston, M.; Leisen, J.; DeMartini, J.; Wyman, C.E.; Ragauskas, A.J. Determination of porosity of lignocellulosic biomass before and after pretreatment by using Simons’ stain and NMR techniques. Bioresour. Technol. 2013, 144, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.N.; Chen, H.-C.; Grethlein, H.E. Comparison of pretreatment methods on the basis of available surface area. Bioresour. Technol. 1992, 39, 155–163. [Google Scholar] [CrossRef]
- Seo, D.-J.; Fujita, H.; Sakoda, A. Structural changes of lignocelluloses by a nonionic surfactant, Tween 20, and their effects on cellulase adsorption and saccharification. Bioresour. Technol. 2011, 102, 9605–9612. [Google Scholar] [CrossRef] [PubMed]
- Esteghlalian, A.R.; Bilodeau, M.; Mansfield, S.D.; Saddler, J.N. Do enzymatic hydrolyzability and Simons’ stain reflect the changes in the accessibility of lignocellulosic substrates to cellulase enzymes? Biotechnol. Prog. 2001, 17, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Allen, T. Surface area and pore size determination. In Particle Size Measurement, 5th ed.; Allen, T., Ed.; Springer: London, UK, 1997; Volume 2, pp. 44–108. ISBN 978-0-412-75330-5. [Google Scholar]
- Guo, J.; Catchmark, J.M. Surface area and porosity of acid hydrolyzed cellulose nanowhiskers and cellulose produced by Gluconacetobacter xylinus. Carbohydr. Polym. 2012, 87, 1026–1037. [Google Scholar] [CrossRef]
- Koo, B.-W.; Min, B.-C.; Gwak, K.-S.; Lee, S.-M.; Choi, J.-W.; Yeo, H.; Choi, I.-G. Structural changes in lignin during organosolv pretreatment of Liriodendron tulipifera and the effect on enzymatic hydrolysis. Biomass Bioenerg. 2012, 42, 24–32. [Google Scholar] [CrossRef]
- Park, S.; Venditti, R.A.; Jameel, H.; Pawlak, J.J. Changes in pore size distribution during the drying of cellulose fibers as measured by differential scanning calorimetry. Carbohydr. Polym. 2006, 66, 97–103. [Google Scholar] [CrossRef]
- Choi, W.-I.; Park, J.-Y.; Lee, J.-P.; Oh, Y.-K.; Park, Y.C.; Kim, J.S.; Park, J.M.; Kim, C.H.; Lee, J.-S. Optimization of NaOH-catalyzed steam pretreatment of empty fruit bunch. Biotechnol. Biofuels 2013, 6, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Mason, G. The effect of pore space connectivity on the hysteresis of capillary condensation in adsorption–desorption isotherms. J. Colloid Interface Sci. 1982, 88, 36–46. [Google Scholar] [CrossRef]
- Thommes, M.; Smarsly, B.; Groenewolt, M.; Ravikovitch, P.I.; Neimark, A.V. Adsorption hysteresis of nitrogen and argon in pore networks and characterization of novel micro- and mesoporous silicas. Langmuir 2006, 22, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Samad, S.K.; Varma, A.K.; Mendhe, V.A. Pore geometrical complexity and fractal facets of Permian shales and coals from Auranga Basin, Jharkhand, India. J. Nat. Gas Sci. Eng. 2018, 52, 25–43. [Google Scholar] [CrossRef]
- Suleimenova, A.; Bake, K.D.; Ozkan, A.; Valenza, J.J., II; Kleinberg, R.L.; Burnham, A.K.; Ferralis, N.; Pomerantz, A.E. Acid demineralization with critical point drying: A method for kerogen isolation that preserves microstructure. Fuel 2014, 135, 492–497. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Rostgaard, J. Critical-point drying versus freeze drying for scanning electron microscopy: A quantitative and qualitative study on isolated hepatocytes. J. Microsc. 1985, 137, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Jie, X.; Cao, Y.; Qin, J.J.; Liu, J.; Yuan, Q. Influence of drying method on morphology and properties of asymmetric cellulose hollow fiber membrane. J. Membr. Sci. 2005, 246, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Hou, Y.; Wu, W.; Niu, M.; Wang, W. Pretreatment of wheat straw using SO2 dissolved in hot water. Bioresour. Technol. 2012, 124, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Ueno, Y.; Saka, S. Thermal reactivities of non-reducing sugars in polyether—Role of intermolecular hydrogen bonding in pyrolysis. J. Anal. Appl. Pyrolysis 2013, 103, 287–292. [Google Scholar] [CrossRef]
- Zhu, Z.; Sathitsuksanoh, N.; Vinzant, T.; Schell, D.J.; McMillan, J.D.; Zhang, Y.-H.P. Comparative study of corn stover pretreated by dilute acid and cellulose solvent–based lignocellulose fractionation: Enzymatic hydrolysis, supramolecular structure, and substrate accessibility. Biotechnol. Bioeng. 2009, 103, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Ishimaru, Y.; Iida, I.; Furuta, Y. Micropore structure of wood: Change in micropore structure accompanied by delignification. J. Wood Sci. 2008, 54, 252–255. [Google Scholar] [CrossRef]





| Pretreatment | Drying Method | Surface Area (m2/g) | Average Pore Diameter (Å) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|
| ALEW | AD 1 | 0.9 | – | 0.005 |
| FD 2 | 2.4 | – | 0.015 | |
| CPD 3 | 161.5 | 61.7 | 0.249 |
| Biomass | Drying Condition | Surface Area (m2/g) | Reference |
|---|---|---|---|
| Spruce 1 | Air drying at 30 °C | 6.3 | [11] |
| Yellow poplar 2 | Freeze drying | 1.8 | [20] |
| Eucalyptus3 | Critical point drying | 58.5 | Current paper |
| Biomass | Measurement Technique | Surface Area (m2/g) | Surface Area Accessible to Cellulose (m2/g) | Reference |
|---|---|---|---|---|
| Corn cob | Solute exclusion | – | 57.4 1 34.1 2 | [7] |
| Protein adsorption | – | 7.7 3 | [33] | |
| Lodgepole pine pulp 4 | Solute exclusion | – | 22.4 | [8] |
| Mixed hard wood 5 | Solute exclusion | 1134 | 25.8 | [15] |
| Filter paper | Protein adsorption | – | 9.8 | [9] |
| Spruce 6 | Dye staining | 57.3 | – | [11] |
| Eucalyptus7 | BET | 58.5 | – | Current paper |
| Pretreatment | Surface Area (m2/g) | Average Pore Diameter (Å) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| Untreated | 0.8 | 34.1 | 0.007 |
| ALEW 1 | 161.5 | 61.7 | 0.249 |
| DA 2 | 58.5 | 70.6 | 0.103 |
| NaOH–steam 3 | 129.9 | 62.1 | 0.202 |
| Pretreatment | Pore Diameter (nm) | |||
|---|---|---|---|---|
| Pd < 2 | 2 < Pd < 5 | 5 < Pd | Total | |
| ALEW | 0.019 | 0.08 | 0.15 (60%) * | 0.249 |
| 1% H2SO4 | 0.003 | 0.03 | 0.07 (68%) | 0.103 |
| NaOH–steam | 0.022 | 0.06 | 0.12 (59%) | 0.202 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.-Y.; Hwang, K.-R.; Park, J.-Y.; Lee, J.-P.; Kim, J.-S.; Lee, J.-S. Critical Point Drying: An Effective Drying Method for Direct Measurement of the Surface Area of a Pretreated Cellulosic Biomass. Polymers 2018, 10, 676. https://doi.org/10.3390/polym10060676
Kang K-Y, Hwang K-R, Park J-Y, Lee J-P, Kim J-S, Lee J-S. Critical Point Drying: An Effective Drying Method for Direct Measurement of the Surface Area of a Pretreated Cellulosic Biomass. Polymers. 2018; 10(6):676. https://doi.org/10.3390/polym10060676
Chicago/Turabian StyleKang, Kyu-Young, Kyung-Ran Hwang, Ji-Yeon Park, Joon-Pyo Lee, Jun-Seok Kim, and Jin-Suk Lee. 2018. "Critical Point Drying: An Effective Drying Method for Direct Measurement of the Surface Area of a Pretreated Cellulosic Biomass" Polymers 10, no. 6: 676. https://doi.org/10.3390/polym10060676
APA StyleKang, K. -Y., Hwang, K. -R., Park, J. -Y., Lee, J. -P., Kim, J. -S., & Lee, J. -S. (2018). Critical Point Drying: An Effective Drying Method for Direct Measurement of the Surface Area of a Pretreated Cellulosic Biomass. Polymers, 10(6), 676. https://doi.org/10.3390/polym10060676