Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
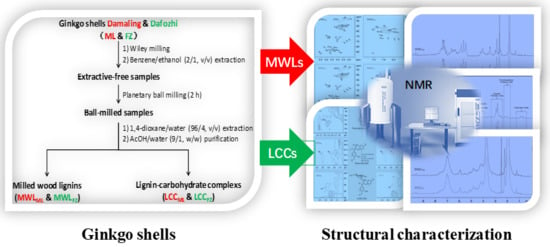
2.2. Isolation of MWLs and LCCs
2.3. Analytical Methods
3. Results and Discussion
3.1. Chemical and Elemental Composition
3.2. Molecular Weight Distribution
3.3. 1H NMR Spectra of MWLs
3.4. 2D HSQC NMR Spectra of MWLs
3.5. 2D HSQC NMR Spectra of LCCs
3.6. Quantification of Lignin Structure and LCC Linkages
3.7. 31P NMR Spectra of MWLs
3.8. Nitrobenzene Oxidation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bidlack, J.E.; Dashek, W.V. Plant cell walls. In Plant Cells and Their Organelles; Dashek, W.V., Miglani, G.S., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 209–238. [Google Scholar]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.X.; He, J.; Narron, R.; Wang, Y.H.; Yong, Q. Characterization of Kraft lignin fractions obtained by sequential ultrafiltration and their potential application as a bio-based component in blends with polyethylene. ACS Sustain. Chem. Eng. 2017, 12, 11770–11779. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Hamel, J.; Katahira, R.; Zhu, H.L. Metal-free aqueous flow battery with novel ultrafiltered lignin as electrolyte. ACS Sustain. Chem. Eng. 2018, 6, 5394–5400. [Google Scholar] [CrossRef]
- Chen, C.L. Nitrobenzene and cupric oxide oxidations. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin, Germany, 1992; pp. 301–321. [Google Scholar]
- Akiyama, T.; Sugimoto, T.; Matsumoto, Y.; Meshitsuka, G. Erythro/threo ratio of beta-O-4 structures as an important structural characteristic of lignin. I: Improvement of ozonation method for the quantitative analysis of lignin side-chain structure. J. Wood Sci. 2002, 48, 210–215. [Google Scholar] [CrossRef]
- Sugimoto, T.; Akiyama, T.; Matsumoto, Y.; Meshitsuka, G. The erythro/threo ratio of β-O-4 structures as an important structural characteristic of lignin. Part 2. Changes in erythro/threo (E/T) ratio of β-O-4 structures during delignification reactions. Holzforschung 2002, 56, 416–421. [Google Scholar] [CrossRef]
- Lu, F.C.; Ralph, J. The DFRC method for lignin analysis. Part 1. A new method for β-aryl ether cleavage: Lignin model studies. J. Agric. Food Chem. 1997, 45, 4655–4660. [Google Scholar] [CrossRef]
- Schäfer, J.; Urbat, F.; Rund, K.; Bunzel, M. A stable-isotope dilution GC-MS approach for the analysis of DFRC (derivatization followed by reductive cleavage) monomers from low-lignin plant materials. J. Agric. Food Chem. 2015, 63, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Capanema, E.A.; Balakshin, M.Y.; Kadla, J.F. A comprehensive approach for quantitative lignin characterization by NMR spectroscopy. J. Agric. Food Chem. 2004, 52, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Mousavioun, P.; Doherty, W.O.S. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.; Hoeger, I.C.; Ferrer, A.; Rencoret, J.; del Rio, J.C.; Kruus, K.; Rahikainen, J.; Kellock, M.; Gutiérrez, A.; Rojas, O.J. Lignin films from spruce, eucalyptus and wheat straw studied with electroacoustic and optical sensors: Effect of composition and electrostatic screening on enzyme binding. BioMacromolecules 2017, 18, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.Y.; Capanema, E.A.; Chang, H.M. MWL fraction with a high concentration of lignin carbohydrate linkages: Isolation and 2D NMR spectroscopic analysis. Holzforschung 2007, 61, 1–7. [Google Scholar] [CrossRef]
- Heikkinen, S.; Toikka, M.M.; Karhunen, T.; Kilpelainen, I.A. Quantitative 2D HSQC (Q-HSQC) via suppression of J-dependence of polarization transfer in NMR spectroscopy: Application to wood lignin. J. Am. Chem. Soc. 2003, 125, 4362–4367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Gellerstedt, G. Achieving quantitative assignment of lignin structure by combining 13C and HSQC NMR techniques. In Proceedings of the 6th European Workshop on Lignocellulosics and Pulp, Bordeaux, France, 3–6 September 2000; pp. 7–10. [Google Scholar]
- Zhang, L.M.; Gellerstedt, G. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn. Reson. Chem. 2007, 45, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yan, H.; Zhang, R.H.; Liu, G.Q.; Chen, C. Characterization and methane production of different nut residue wastes in anaerobic digestion. Renew. Energy 2018, 116, 835–841. [Google Scholar] [CrossRef]
- Björkman, A. Studies on finely divided wood. Part I. Extraction of lignin with neutral solvents. Sven Papperstidn 1956, 59, 477–485. [Google Scholar]
- Gu, F.; Wu, W.J.; Wang, Z.G.; Yokoyama, T.; Jin, Y.C. Effect of complete dissolution in LiCl/DMSO on the isolation and characteristics of lignin from wheat straw internode. Ind. Crops Prod. 2015, 74, 703–711. [Google Scholar] [CrossRef]
- Huang, C.X.; He, J.; Du, L.T.; Min, D.Y.; Yong, Q. Structural characterization of the lignins from the green and yellow bamboo of bamboo culm (Phyllostachys pubescens). J. Wood Chem. Technol. 2016, 36, 157–172. [Google Scholar] [CrossRef]
- Lundquist, K. Proton (1H) NMR spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin, Germany, 1992; pp. 242–249. [Google Scholar]
- Wiselogel, A.; Tyson, S.; Johnson, D. Biomass feedstock resources and composition. In Handbook on Bioethanol: Production and Utilization; Wyman, C.E., Ed.; Taylor & Francis: Washington, DC, USA, 1996; pp. 105–118. [Google Scholar]
- Timell, T.E. Studies on Ginkgo biloba, L. 1. General characteristics and chemical composition. Svensk Papperstidning 1960, 63, 652–657. [Google Scholar]
- Van Beek, T.A.; Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Cavaleiro, J.A.S.; Domingues, F.M.J.; Silvestre, A.J.D.; Evtuguin, D.; Neto, C.P. Structural characterization of the lignin from the nodes and internodes of Arundo donax reed. J. Agric. Food Chem. 2000, 48, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef] [PubMed]
- del Río, J.C.; Prinsen, J.; Rencoret, J.; Nieto, L.; Jiménez-Barbero, J.; Ralph, J.; Martínez, A.T.; Gutiérrez, A. Structural characterization of the lignin in the cortex and pith of elephant grass (Pennisetum purpureum) Stems. J. Agric. Food Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rencoret, J.; Prinsen, P.; Gutieŕrez, A.; Martıńez, A.T.; del Río, J.C. Isolation and structural characterization of the milled wood lignin, dioxane lignin, and cellulolytic lignin preparations from brewer′s spent grain. J. Agric. Food Chem. 2015, 63, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ralph, J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org. Biomol. Chem. 2010, 8, 576–591. [Google Scholar] [CrossRef] [PubMed]
- del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralph, J.; Hatfield, R.D.; Piquemal, J.; Yahiaoui, N.; Pean, M.; Lapierre, C.; Boudet, A.M. NMR characterization of altered lignins extracted from tobacco plants down-regulated for lignification enzymes cinnamyl-alcohol dehydrogenase and cinnamyl-CoA reductase. Proc. Natl. Acad. Sci. USA 1998, 95, 12803–12808. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Lignin biochemistry: Biosynthesis and biodegradation. Wood Sci. Technol. 1990, 24, 23–63. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- You, T.T.; Zhang, L.M.; Zhou, S.K.; Xu, F. Structural elucidation of lignin–carbohydrate complex (LCC) preparations and lignin from Arundo donax Linn. Ind. Crops Prod. 2015, 71, 65–74. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of lignin structures and lignin-carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; He, J.; Li, X.; Min, D.Y.; Yong, Q. Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin–carbohydrates complexes. Bioresour. Technol. 2015, 192, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.Y.; Capanema, E.A.; Gracz, H.; Chang, H.M.; Jameel, H. Quantification of lignincarbohydrate linkages with high resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Tokimatsu, T.; Umezawa, T.; Shimada, M. Synthesis of four diastereomeric lignin carbohydrate complexes (LCC) model compounds composed of a β-O-4 lignin model linked to methyl β-d-glucose. Holzforschung 1996, 50, 156–160. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.C.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Akim, L.G.; Colodette, J.L.; Argyropoulos, D.S. Factors limiting oxygen delignification of kraft pulp. Can. J. Chem. 2001, 79, 201–210. [Google Scholar] [CrossRef]
- Tamai, A.; Goto, H.; Akiyama, T.; Matsumoto, Y. Revisiting alkaline nitrobenzene oxidation: Quantitative evaluation of biphenyl structures in cedar wood lignin (Cryptomeria japonica) by a modified nitrobenzene oxidation method. Holzforschung 2015, 69, 951–958. [Google Scholar] [CrossRef]
- Min, D.Y.; Xiang, Z.Y.; Liu, J.; Jameel, H.; Chiang, V.; Jin, Y.C.; Chang, H.M. Improved protocol for alkaline nitrobenzene oxidation of woody and non-woody biomass. J. Wood Chem. Technol. 2015, 35, 52–61. [Google Scholar] [CrossRef]
- Coleman, H.D.; Park, J.Y.; Nair, R.; Chapple, C.; Mansfield, S.D. RNAi-mediated suppression of p-coumaroyl-CoA 3′-hydroxylase in hybrid poplar impacts lignin deposition and soluble secondary metabolism. Proc. Natl. Acad. Sci. USA 2008, 105, 4501–4506. [Google Scholar] [CrossRef] [PubMed]








| Samples 1 | Carbohydrates | Lignin | Ash | Yield 2 | |||
|---|---|---|---|---|---|---|---|
| Glucan | Xylan | Arabinan + Mannan | Klason | Acid Soluble | |||
| ML | 21.3 ± 0.1 | 19.9 ± 0.7 | 1.9 ± 0.3 | 42.4 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.1 | - |
| FZ | 20.0 ± 0.4 | 20.2 ± 0.2 | 1.5 ± 0.1 | 42.0 ± 0.2 | 0.7 ± 0.2 | 0.9 ± 0.1 | - |
| MWLML | 0.2 ± 0.0 | 1.1 ± 0.0 | 0.3 ± 0.0 | 93.6 ± 1.5 | 3.9 ± 0.0 | 0.0 | 17.5 |
| MWLFZ | 0.9 ± 0.1 | 2.9 ± 0.2 | 0.4 ± 0.1 | 90.3 ± 1.8 | 3.9 ± 0.1 | 0.0 | 15.3 |
| LCCML | 12.0 ± 0.7 | 17.7 ± 0.7 | 3.1 ± 0.5 | 24.5 ± 1.5 | 17.9 ± 0.2 | 1.4 ± 0.2 | N.D. |
| LCCFZ | 13.7 ± 0.1 | 17.8 ± 0.4 | 2.7 ± 0.1 | 24.1 ± 1.5 | 17.5 ± 0.1 | 1.6 ± 0.1 | N.D. |
| Samples | C | H | N | S | O | Mw | Mn | Mw/Mn |
|---|---|---|---|---|---|---|---|---|
| MWLML | 62.5 | 5.5 | 1.5 | 0.4 | 30.1 | 12,130 | 3800 | 3.19 |
| MWLFZ | 62.1 | 5.6 | 1.5 | 0.3 | 30.5 | 11,550 | 3930 | 2.94 |
| Label | δC/δH (ppm) | Assignment |
|---|---|---|
| Est | 65-62/4.5-4.0 | C-H in γ-ester linkages |
| X5 | 62.6/3.40 | C5-H5 in β-d-xylopyranoside |
| M22 | 70.3/5.27 | C2-H2 in 2-O-acetyl-β-d-mannopyranoside |
| X2 | 72.6/3.05 | C2-H2 in β-d-xylopyranoside |
| X22 | 72.8/4.43 | C2-H2 in 2-O-acetyl-β-d-xylopyranoside |
| M33 | 73.6/4.93 | C3-H3 in 3-O-acetyl-β-d-mannopyranoside |
| X3 | 73.8/3.25 | C3-H3 in β-d-xylopyranoside |
| X33 | 73.9/4.61 | C3-H3 in 3-O-acetyl-β-d-xylopyranoside |
| X4 | 75.4/3.53 | C4-H4 in β-d-xylopyranoside |
| U4 | 81.1/3.10 | C4-H4 in 4-O-methyl-α-d-glucuronic acid |
| BE2 | 81.4/5.04 | Cα-Hα in benzyl ether (primary OH) linkages |
| BE1 | 81.6/4.64 | Cα-Hα in benzyl ether (secondary OH) linkages |
| αX1(R) | 91.9/4.89 | C1-H1 in (1→4)-α-d-xylopyranoside (R) |
| βX1(R) | 96.8/4.26 | C1-H1 in (1→4)-β-d-xylopyranoside (R) |
| U1 | 97.0/5.07 | C1-H1 in 4-O-methyl-α-d-glucuronic acid |
| X231 | 98.3/4.72 | C1-H1 in 2,3-O-acetyl-β-d-xylopyranoside |
| X21 | 99.8/4.52 | C1-H1 in 2-O-acetyl-β-d-xylopyranoside |
| X31 | 101.3/4.28 | C1-H1 in 3-O-acetyl-β-d-xylopyranoside |
| PhGlc3 | 101.5/4.90 | C3-H3 in phenyl glycoside linkages |
| X1/Glc1 | 103.0/4.31 | C1-H1 in β-d-xylopyranoside/β-d-glucopyranoside |
| Range (ppm) | Assignment | Amount | |
|---|---|---|---|
| ML | FZ | ||
| Lignin characteristic 1 | |||
| 199–196 | Cα = O except G′ | 0.07 | 0.05 |
| 196–193 | C = O in Cα = O/β-O-4′ (A′), F, J | 0.08 | 0.07 |
| 193–190 | Ar-CHO | 0.07 | 0.07 |
| 182–179 | C4 in F (β-1′) | 0.03 | 0.03 |
| 175–168.5 | aliphatic COOR | 0.07 | 0.09 |
| 168.5–166 | conjugated COOR | 0.02 | 0.03 |
| 159–151 | Cα in J, C3, 6 in F, C4 in conjugated CO/COOR etherified units | 0.29 | 0.25 |
| 144.5–142.5 | C3 in B (β-β′) | 0.20 | 0.25 |
| 57–54 | –OCH3, C1 in F | 1.05 | 1.08 |
| 54–52 | Cβ in B and C (β-β′, β-5′) | 0.18 | 0.16 |
| Clusters 1 | |||
| 163–142 | aromatic C-O | 1.85 | 1.79 |
| 142–125 | aromatic C-C | 1.52 | 1.56 |
| 125–102 | aromatic C-H | 2.62 | 2.67 |
| 90–58 | Alk-O- | 2.23 | 2.36 |
| 90–77 | Alk-O-Ar, α-O-Alk | 0.83 | 0.83 |
| 77–65 | γ-O-Alk, secondary OH | 0.68 | 0.75 |
| Interunit linkages and structural units 2 | |||
| 111.0/6.98 | guaiacyl units (G) | 99 | 99 |
| 128.0/7.20 | p-hydroxyphenyl units (H) | 1 | 1 |
| 71.2/4.74 | β-O-4′ alkyl ether linkages (A′) | 40 | 41 |
| 87.0/5.45 | phenylcoumaran substructures (B) | 12 | 14 |
| 84.9/4.63 | resinol substructures (C) | 3 | 4 |
| 83.3/4.83 | dibenzodioxocin substructures (D) | 1 | 2 |
| 81.4/5.03 | spirodienone substructures (F) | <1 | <1 |
| LCC linkages 3 | |||
| 104.0–98.0/5.30–4.50 | PhGlc | 0.035 | 0.027 |
| 82.5–80.0/5.30–4.30 | BE | 0.008 | 0.008 |
| 66.0–62.0/4.50–4.00 | Est | 0.026 | 0.039 |
| Samples | Aliphatic OH | Phenolic OH | C + S | G | H | COOH |
|---|---|---|---|---|---|---|
| MWLML | 4.44 | 1.38 | 0.42 | 0.78 | 0.18 | 0.16 |
| MWLFZ | 4.30 | 1.23 | 0.39 | 0.69 | 0.15 | 0.18 |
| Samples | Yield (mmol/g-lignin) | V/S/H 1 | |||
|---|---|---|---|---|---|
| V | S | H | Total | ||
| ML | 1.65 ± 0.02 | 0.02 ± 0.00 | 0.11 ± 0.01 | 1.79 ± 0.01 | 92/1/7 |
| FZ | 1.47 ± 0.05 | 0.02 ± 0.00 | 0.15 ± 0.01 | 1.64 ± 0.02 | 90/1/9 |
| LCCML | 2.84 ± 0.09 | 0.02 ± 0.00 | 0.14 ± 0.00 | 2.25 ± 0.03 | 95/1/4 |
| LCCFZ | 2.14 ± 0.00 | 0.02 ± 0.00 | 0.10 ± 0.00 | 3.00 ± 0.00 | 95/1/4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Zhang, Y.; Guo, T.; Zhao, H.; Jin, Y. Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy. Polymers 2018, 10, 736. https://doi.org/10.3390/polym10070736
Jiang B, Zhang Y, Guo T, Zhao H, Jin Y. Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy. Polymers. 2018; 10(7):736. https://doi.org/10.3390/polym10070736
Chicago/Turabian StyleJiang, Bo, Yu Zhang, Tianyu Guo, Huifang Zhao, and Yongcan Jin. 2018. "Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy" Polymers 10, no. 7: 736. https://doi.org/10.3390/polym10070736
APA StyleJiang, B., Zhang, Y., Guo, T., Zhao, H., & Jin, Y. (2018). Structural Characterization of Lignin and Lignin-Carbohydrate Complex (LCC) from Ginkgo Shells (Ginkgo biloba L.) by Comprehensive NMR Spectroscopy. Polymers, 10(7), 736. https://doi.org/10.3390/polym10070736