Novel Hydrophobic Associating Polymer with Good Salt Tolerance
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Raw Materials
2.2. Synthesis of DiPHAM
3. Methods
3.1. Characterization
3.2. DLS Measurements
3.3. Rheological Properties Evaluation
3.4. Microstructure Analysis
4. Results and Discussion
4.1. Characterization of DiPHAM
4.2. Critical Association Concentration
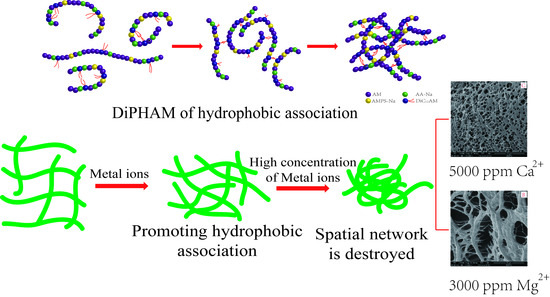
4.3. Effects of Metal Ions
4.4. Effect on Structure of DiPHAM
4.4.1. Viscoelasticity Properties
4.4.2. Thixotropy
4.4.3. Microstructure Observation
4.5. Effect of Metal Ions on Rheological Properties
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Wang, Z.M.; Ye, Z.B.; Han, L.J. The solution behavior of hydrophobically associating zwitterionic polymer in salt water. J. Appl. Polym. Sci. 2014, 131, 1–15. [Google Scholar] [CrossRef]
- Lin, C.A.; Sperling, R.A.; Li, J.K.; Yang, T.Y.; Li, P.Y.; Zanella, M.; Chang, W.H.; Parak, W.J. Design of an amphiphilic polymer for nanoparticle coating and functionalization. Small 2008, 4, 301. [Google Scholar] [CrossRef]
- Kuang, W.; Gao, C. Synthesis and characterization of novel twin-tailed hydrophobically associated copolymers and their applications to Cr(III) removal from aqueous solutions. J. Appl. Polym. Sci. 2015, 131, 8558–8572. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Nishio, R.; Hirano, T.; Koshi, Y.; Nukiwa, M.; Koiwa, M.; Michizoe, J.; Ida, N. Controlled release of protein drugs from newly developed amphiphilic polymer-based microparticles composed of nanoparticles. J. Control. Release 2010, 142, 8–13. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.L.; Johnson, C.B. Structurally Tailored Macromolecules for Mobility Control in Enhanced Oil Recovery. In Water-Soluble Polymers for Petroleum Recovery; Springer: Boston, MA, USA, 1988; pp. 161–180. [Google Scholar]
- Klucker, R.; Munch, J.P.; Schosseler, F. Combined Static and Dynamic Light Scattering Study of Associating Random Block Copolymers in Solution. Macromolecules 1997, 30, 3839–3848. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tomatsu, I.; Hashidzume, A.; Morishima, Y. Associative Properties in Water of Copolymers of Sodium 2-(Acrylamido)-2-methylpropanesulfonate and Methacrylamides Substituted with Alkyl Groups of Varying Lengths. Macromolecules 2000, 33, 7852–7861. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Chen, M. The Length of Hydrophobic Chain in Amphiphilic Polypeptides Regulates the Efficiency of Gene Delivery. Polymers 2018, 10, 379. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Yan, D. A controlled release system for simultaneous promotion of gene transfection and antitumor effects. RSC Adv. 2014, 4, 64596–64600. [Google Scholar] [CrossRef]
- Shalaby, S.W.; McCormick, C.L.; Butler, G.B. Water-soluble polymers: Synthesis, solution properties, and applications. Carbohydr. Polym. 1991, 467, 197–212. [Google Scholar]
- Dusseault, M.; Li, S.; Han, H.; Li, J.; Wu, H. Flooding Thin Low-Permeability Layers with a New Salt-Resistant, Medium-Molecular-Weight Polymer; SPE 109627; Society of Petroleum Engineers: Richardson, TX, USA, 2007. [Google Scholar]
- Jiang, G.; Liu, F. Effect of Mineral Salts on Steady Rheological Properties of Nonionic Hydrophobically Modified Polyacrylamide and Its Stress-Relaxation Behavior. J. Macromol. Sci. A 2013, 50, 1209–1217. [Google Scholar] [CrossRef]
- Zhu, Z.; Kang, W.; Sarsenbekuly, B.; Yang, H.; Dai, C.; Yang, R.; Fan, H. Preparation and solution performance for the amphiphilic polymers with different hydrophobic groups. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Sarsenbekuly, B.; Kang, W.; Fan, H.; Yang, H.; Dai, C.; Zhao, B.; Aidarova, S.B. Study of salt tolerance and temperature resistance of a hydrophobically modified polyacrylamide based novel functional polymer for EOR. Colloids Surf. A 2017, 514, 91–97. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Wang, B.; Zheng, C.; Huang, Z. Self-assembling transition behavior of a hydrophobic associative polymer based on counterion and pH effects. Colloids Surf. A 2016, 490, 1–8. [Google Scholar] [CrossRef]
- Hill, A.; Candau, F.; Selb, J. Properties of hydrophobically associating polyacrylamides: Influence of the method of synthesis. Macromolecules 1993, 26, 4521–4532. [Google Scholar] [CrossRef]
- Hiorns, R.C.; Boucher, R.J.; Duhlev, R.; Hellwich, K.-H.; Hodge, P.; Jenkins, A.D.; Jones, R.G.; Kahovec, J.; Moad, G.; Ober, C.K.; et al. A brief guide to polymer nomenclature (IUPAC Technical Report). Pure Appl. Chem. 2012, 84, 2167–2169. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Huang, R.; Xu, J. Characterization, Solution Behavior, and Microstructure of a Hydrophobically Associating Nonionic Copolymer. J. Solut. Chem. 2008, 37, 1227–1243. [Google Scholar] [CrossRef]
- Qi, G.; Li, H.; Zhu, R.; Zhang, Z.; Zhou, L.; Kuang, J. Synthesis, Characterization, and Solution Behavior of a Long-Chain Hydrophobic Association Anionic Acrylamide/2-Acrylamido-2-Methylpropanesulfonic Acid/n-Octyl Acrylate Terpolymers. Arab. J. Sci. Eng. 2017, 42, 2425–2432. [Google Scholar] [CrossRef]
- Peng, S.; Wu, C. Light Scattering Study of the Formation and Structure of Partially Hydrolyzed Poly(acrylamide)/Calcium(II) Complexes. Macromolecules 1999, 32, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Brown, W.; Konak, C. Dynamic light scattering study of the sol-gel transition. Macromolecules 1991, 24, 6839–6842. [Google Scholar] [CrossRef]
- Ritacco, H.; Kurlat, D.H. Critical aggregation concentration in the PAMPS (10%)/DTAB system. Colloids Surf. A 2003, 218, 27–45. [Google Scholar] [CrossRef]
- Härtel, A.; Janssen, M.; Samin, S.; van Roij, R. Fundamental measure theory for the electric double layer: Implications for blue-energy harvesting and water desalination. J. Phys. Condens. Matter 2015, 27, 194129. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.J.; Kremer, K. The nature of flexible linear polyelectrolytes in salt free solution: A molecular dynamics study. J. Chem. Phys. 1995, 103, 1669–1690. [Google Scholar] [CrossRef]
- Feng, J.G.; Lu, F.S.; Chen, T.T.; Zhang, S.Q.; Li, H. Effect of Copolymer Dispersant on the Dispersion Stability of Flufenoxuron Suspension Concentrate. Chem. J. Chin. Univ. 2010, 31, 1386–1390. [Google Scholar]
- Iv, C.F.Z.; Saville, D.A. The interpretation of electrokinetic measurements using a dynamic model of the stern layer: II. Comparisons between theory and experiment. J. Colloid Interface Sci. 1986, 114, 45–53. [Google Scholar]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wang, W.; Yang, M. Synthesis and solution properties of an associative polymer with excellent salt-thickening. J. Appl. Polym. Sci. 2012, 125, 4049–4059. [Google Scholar] [CrossRef]
- Chollakup, R.; Beck, J.B.; Dirnberger, K.; Tirrell, M.; Eisenbach, C.D. Polyelectrolyte Molecular Weight and Salt Effects on the Phase Behavior and Coacervation of Aqueous Solutions of Poly (acrylic acid) Sodium Salt and Poly (allylamine) Hydrochloride. Macromolecules 2013, 46, 2376–2390. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Kamal, H.; Hegazy, E.S.A.; Sharada, H.M.; Elhalim, S.A.A.; Lotfy, S.; Mohamed, R.D. Immobilization of glucose isomerase onto radiation synthesized P(AA-co-AMPS) hydrogel and its application. J. Radiat. Res. Appl. Sci. 2014, 7, 154–162. [Google Scholar] [CrossRef]
- Xia, L.H. Effect of metal cation and degree of mineralization on the viscosity of ultra-high-molecular-weight polymer solution. Chem. Eng. Des. Commun. 2017, 43, 205–206. [Google Scholar]
- Wang, J.J.; Lv, J.F.; Cao, P.X.; Zhang, M.L.; Gao, L.J.; Lei, L.; Ren, Y.X.; Hou, X.Y. Effect of Metal Ions on the Structures of Coordination Polymers Based on Biphenyl-2,2′,4,4′-Tetracarboxylate. Z. Anorg. Allg. Chem. 2011, 637, 1585–1589. [Google Scholar] [CrossRef]
- Rivas, B.L.; Moreno-Villoslada, I. Effect of the Polymer Concentration on the Interactions of Water-Soluble Polymers with Metal Ions. Chem. Lett. 2001, 78, 166–167. [Google Scholar] [CrossRef]
- Murugaboopathy, S.; Matsuoka, H. Surface Active to Non-Surface Active Transition and Micellization Behaviour of Zwitterionic Amphiphilic Diblock Copolymers: Hydrophobicity and Salt Dependency. Polymers 2017, 9, 412. [Google Scholar] [CrossRef]
- Jing, X.; Gong, W.; Feng, Z.; Meng, X.; Zheng, B. Influence of comb-like copolymer dispersants with different molecular structures on the performance of CaCO3 suspension in organic system. J. Dispers. Sci. Technol. 2016, 38, 1311–1318. [Google Scholar] [CrossRef]
- Castelnovo, M.; Joanny, J.F. Complexation between oppositely charged polyelectrolytes: Beyond the Random Phase Approximation. Eur. Phys. J. E 2001, 6, 377–386. [Google Scholar] [CrossRef]
- Kudlay, A.; Ermoshkin, A.V.; de la Cruz, M.O. Complexation of Oppositely Charged Polyelectrolytes: Effect of Ion Pair Formation. Macromolecules 2004, 37, 9231–9241. [Google Scholar] [CrossRef]
- Netz, R.R.; Andelman, D. Neutral and charged polymers at interfaces. Phys. Rep. 2002, 380, 1–95. [Google Scholar] [CrossRef]
- And, A.C.; Chowdhuri, S. Pressure Effects on the Dynamics and Hydrogen Bond Properties of Aqueous Electrolyte Solutions: The Role of Ion Screening. J. Phys. Chem. B 2002, 106, 6779–6783. [Google Scholar]
- Crozier, P.S.; Rowley, R.L.; Henderson, D. Molecular-dynamics simulations of ion size effects on the fluid structure of aqueous electrolyte systems between charged model electrodes. J. Chem. Phys. 2001, 114, 7513–7517. [Google Scholar] [CrossRef]
- Kaewsaiha, P.; Matsumoto, K.; Matsuoka, H. Non-surface activity and micellization of ionic amphiphilic diblock copolymers in water. Hydrophobic chain length dependence and salt effect on surface activity and the critical micelle concentration. Langmuir 2005, 21, 9938–9945. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.A. Thixotropy—A review. J. Non-Newton. Fluid Mech. 1997, 70, 1–33. [Google Scholar] [CrossRef]
- Dullaert, K.; Mewis, J. A model system for thixotropy studies. Rheol. Acta 2005, 45, 23–32. [Google Scholar] [CrossRef]
- Mujumdar, A.; Beris, A.N.; Metzner, A.B. Transient phenomena in thixotropic systems. J. Non-Newton. Fluid Mech. 2002, 102, 157–178. [Google Scholar] [CrossRef]
- Rubio-Hernández, F.J.; Velázquez-Navarro, J.F.; Galindo-Rosales, F.J. Rheological characterization of a time dependent fresh cement paste. Mech. Time-Depend. Mater. 2009, 13, 199–206. [Google Scholar] [CrossRef]
- Chhabra, R.P. Non-Newtonian Fluids: An Introduction. In Rheology of Complex Fluids; Springer: New York, NY, USA, 2010; pp. 3–34. [Google Scholar]
- Wallevik, J.E. Microstructure-Rheology: Thixotropy and workability loss. Nord. Concr. Res. 2004, 31, 16–29. [Google Scholar]
- Spinelli, L.S.; Pires, R.V.; Barboza, E.M.; Louvisse, A.M.T.; Aquino, A.S.; Lucas, E.F. Influence of polymer bases on the synergistic effects obtained from mixtures of additives in the petroleum industry: Performance and residue formation. J. Petrol. Sci. Eng. 2007, 58, 111–118. [Google Scholar] [CrossRef]
- Lucas, E.F.; Spinelli, L.S.; Khalil, C.N. Polymers Applications in Petroleum Production; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 129, pp. 1344–1348. [Google Scholar]






















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Tan, H.; Yang, B.; Zhang, W.; Yang, X.; Zhang, Y.; Zhang, H. Novel Hydrophobic Associating Polymer with Good Salt Tolerance. Polymers 2018, 10, 849. https://doi.org/10.3390/polym10080849
Mao J, Tan H, Yang B, Zhang W, Yang X, Zhang Y, Zhang H. Novel Hydrophobic Associating Polymer with Good Salt Tolerance. Polymers. 2018; 10(8):849. https://doi.org/10.3390/polym10080849
Chicago/Turabian StyleMao, Jincheng, Hongzhong Tan, Bo Yang, Wenlong Zhang, Xiaojiang Yang, Yang Zhang, and Heng Zhang. 2018. "Novel Hydrophobic Associating Polymer with Good Salt Tolerance" Polymers 10, no. 8: 849. https://doi.org/10.3390/polym10080849
APA StyleMao, J., Tan, H., Yang, B., Zhang, W., Yang, X., Zhang, Y., & Zhang, H. (2018). Novel Hydrophobic Associating Polymer with Good Salt Tolerance. Polymers, 10(8), 849. https://doi.org/10.3390/polym10080849